ICSE Class 10 students can refer to ICSE Class 10 Analytical Chemistry Notes given below which have been prepared as per the latest syllabus and guidelines issued by ICSE Board. All chapter wise revision notes for ICSE Class 10 Chemistry have been prepared by teachers have strong understanding of Chemistry. Read the notes prior to the exams to get better marks in exams
Analytical Chemistry ICSE Class 10 Chemistry Revision Notes
Students can refer to the quick revision notes prepared for Chapter Analytical Chemistry in Class 10 ICSE. These notes will be really helpful for the students giving the Chemistry exam in ICSE Class 10. Our teachers have prepared these concept notes based on the latest ICSE syllabus and ICSE books issued for the current academic year.
Revision Notes ICSE Class 10 Chemistry Analytical Chemistry
Please refer to the detailed notes below
Introduction
The determination of the chemical components in a given sample is called analysis. Types of Analysis
• Qualitative Analysis: It involves the identification of unknown substances.
• Quantitative Analysis: It involves the identification of the composition of a mixture.
Reagents
• Reagent: It is a substance which reacts with another substance.
• Alkalis are important laboratory reagents.
• Sodium hydroxide and ammonium hydroxide are the most commonly used alkalis, which give
characteristic tests with various metal cations, and thus, a cation can be identified.
Colours of Salts and their Solutions
Salts of representative elements (normal elements), i.e. the elements of Group IA to Group VII A are
generally colourless.
Salts of transition elements, i.e. salts of elements of Group IB to VIIB and Group VIII are generally
coloured.

Precipitation
It is the process of formation of an insoluble solid when solutions are mixed. The solid thus formed is
known as a precipitate.
Action of Sodium Hydroxide Solution on Metallic Salt Solutions

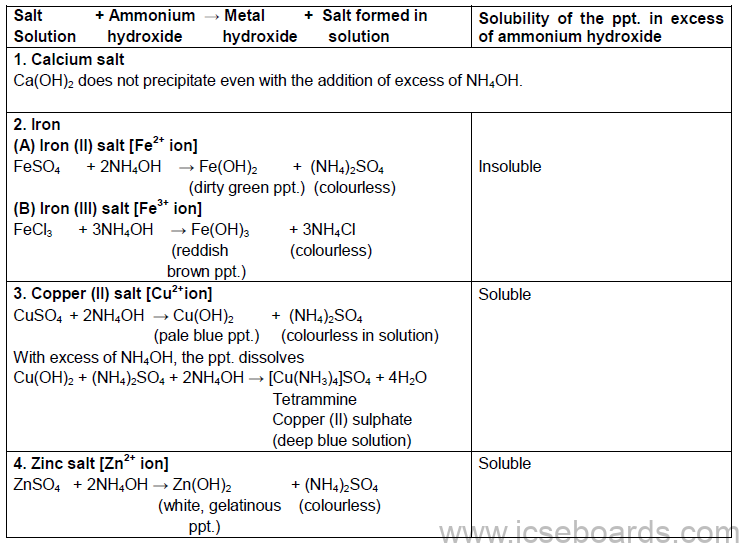
Action of Ammonium Hydroxide on certain Salt Solutions
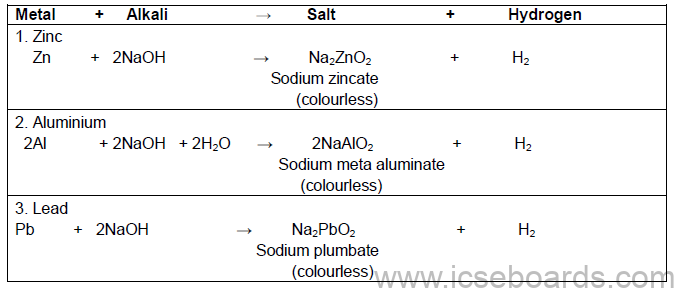
Action of Alkalis on certain Metals
Action of Alkalis on Metal Oxides
Amphoteric oxides and hydroxides: Compounds which react with both acids and alkalis to form salt
and water are called amphoteric oxides and hydroxides.

Analytical Chemistry
In the qualitative analysis of compounds, their colour helps in their identification. The table given below shows some examples of colourless and coloured ions.


Chemical reactions of the soluble salt solutions with NaOH and NH4OH
Some soluble salts (except sodium and potassium) react with sodium hydroxide and ammonium hydroxide to form insoluble precipitates.
Reactions with sodium hydroxide solution
Aqueous ferrous sulphate (green in colour) reacts with NaOH to form iron (II) hydroxide, which is insoluble in alkali.
FeSO4 + 2NaOH → Na2SO4 + Fe(OH)2
Other such reactions are shown below:

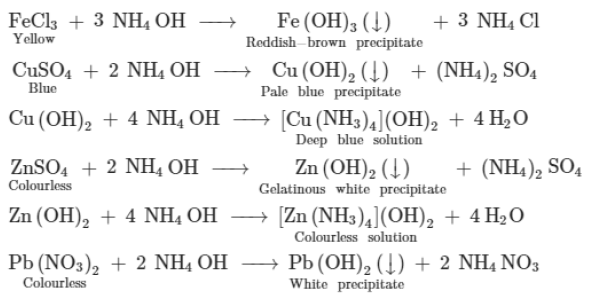
Reactions with ammonium hydroxide: Aqueous ferrous sulphate (green in colour) reacts with NH4OH to form iron (II) hydroxide, which is insoluble in excess of ammonium hydroxide.
FeSO4 + 2NH4 OH → (NH4)2SO4 + FE(OH)2
Other such reactions are shown below:
Amphoteric nature of zinc and aluminium metals, their oxides and hydroxides
• Amphoteric nature of zinc and aluminium metals: As zinc and aluminium metals displace hydrogen from the acids as well as alkali, therefore, they are amphoteric in nature.

• Amphoteric nature of zinc and aluminium oxides: As the oxides of zinc and aluminium react with acids as well as alkalies to form salt and water, they are amphoteric in nature.

• Amphoteric nature of hydroxides of zinc and aluminium metals: As the hydroxides of zinc and aluminium react with acids as well as alkalies to form salt and water as the only products, therefore, they are amphoteric in nature.

• Amphoteric nature of lead oxide: As the oxide of lead react both with hydrochloric acid and sodium hydroxide, it is amphoteric in nature.
pbO + 2HCI → pbCI2 + H20
pbO + 2NaOH + H2O → Na [Pb(OH)4]
• Amphoteric nature of lead hydroxide: As the hydroxide of lead react both with hydrochloric acid and sodium hydroxide, it is amphoteric in nature.
pb(OH)2 + 2HCI → pbCI2 + 2H20
pb(OH)2 + 2NaOH → Na2 [Pb(OH)4