ICSE Class 10 students can refer to ICSE Class 10 Mole Concept and Stoichiometry Notes given below which have been prepared as per the latest syllabus and guidelines issued by ICSE Board. All chapter wise revision notes for ICSE Class 10 Chemistry have been prepared by teachers have strong understanding of Chemistry. Read the notes prior to the exams to get better marks in exams
Mole Concept and Stoichiometry ICSE Class 10 Chemistry Revision Notes
Students can refer to the quick revision notes prepared for Chapter Mole Concept and Stoichiometry in Class 10 ICSE. These notes will be really helpful for the students giving the Chemistry exam in ICSE Class 10. Our teachers have prepared these concept notes based on the latest ICSE syllabus and ICSE books issued for the current academic year.
Revision Notes ICSE Class 10 Chemistry Mole Concept and Stoichiometry
Please refer to the detailed notes below
Gas Laws
Boyle’s Law
The volume of a given mass of a dry gas is inversely proportional to its pressure at a constant
temperature.
P1VI = P2V2 = k at constant temperature
Charles’s Law
The volume of a given mass of a dry gas is directly proportional to its absolute temperature if the pressure
is kept constant.

Gas Equation
The volume of a given mass of a dry gas is inversely proportional to the pressure and directly proportional
to the absolute temperature.

Standard or Normal Temperature and Pressure
For temperature: 0°C or 273 K
For pressure: 760 mm or 76 cm of Hg
Gay-Lussac’s law of combining volumes
At the same temperature and pressure, the volume of gases taking part in a chemical reaction as either
reactants or products bears a whole number ratio to one another.

The ratio of reacting gases and products is 1:1:2, which is a simple ratio.
Avogadro’s Law
Under the same conditions of temperature and pressure, equal volumes of all the gases contain the same
number of molecules.
Example: A molecule of NH3 is made of one atom of nitrogen and three atoms of hydrogen.
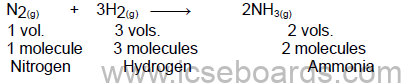
Atomicity
The number of atoms in a molecule of an element is called its atomicity.
a. Monatomic: It is composed of only one atom.
Examples: Inert gases such as Helium, Neon etc.
b. Diatomic: It is composed of two identical atoms.
Examples: H2, O2, Cl2 etc.
c. Triatomic: It is composed of three identical atoms.
Example: Ozone (O3)
d. Tetratomic: It is composed of four identical atoms.
Example: Phosphorus (P4)
e. Octatomic: It is composed of eight identical atoms.
Example: Sulphur (S8)
Atomic Mass or Relative Atomic Mass
It is the number which represents how many times one atom of an element is heavier than 1/12th the mass
of an atom of carbon-12 (12C).

Molecular Mass or Relative Molecular Mass
It is the number which represents how many times one molecule of an element is heavier than 1/12th the
mass of an atom of carbon-12 (12C).
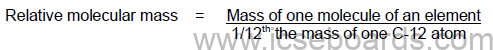
Gram Atomic Mass
The atomic mass of an element expressed in gram is called gram atomic mass.
Example: Gram atomic mass of oxygen is 16 gram.
Gram Molecular Mass
The molecular mass of a substance expressed in gram is called gram molecular mass or molar mass.
Example: Gram molecular mass of water is 18 gram.
Mole Concept
A mole is a collection of 6.022 × 1023 particles.
A mole is defined as the amount of a substance containing elementary particles such as atoms, molecules
or ions in 12 gram of carbon-12 (12C).
Avogadro’s Number
It is defined as the number of atoms present in 12 gram of C-12 isotope, i.e. 6·023 × 1023 atoms. It is
denoted by NA or L.
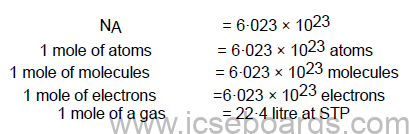
Applications of Avogadro’s Law
i. It explains Gay-Lussac’s law.
ii. It determines atomicity of the gases.
iii. It determines the molecular formula of a gas.
iv. It determines the relation between molecular mass and vapour density.
v. It gives the relationship between gram molecular mass and gram molar volume.
Relative Vapour Density (VD)
Relative vapour density is the ratio between the masses of equal volumes of a gas (or vapour) and
hydrogen under the same conditions of temperature and pressure.

Important Formulae

Percentage Composition
The percentage by weight of each element present in a compound is called percentage composition of the
compound

Empirical Formula
It is the chemical formula which gives the simplest ratio in whole numbers of atoms of different elements
present in one molecule of the compound.
Empirical Formula Mass
It is the sum of atomic masses of various elements present in the empirical formula.
Empirical Formula Weight (EFW)
The empirical formula weight is the atomic masses of the elements present in the empirical formula.
EFW of H2O2 = 2 × (H) + 2 × (0)
= 2 × 1 + 2 × 16
= 34 amu
Molecular Formula
It denotes the actual number of atoms of different elements present in one molecule of the compound.
Molecular formula = Empirical formula × n

Relationship between Empirical Formula and Molecular Formula
Molecular formula = Empirical formula × n
Where ‘n’ is a positive whole number

Chemical Equation
A shorthand notation of describing an actual chemical reaction in terms of symbols and formula along with
the number of atoms and molecules of the reactants and products is called a chemical equation.
A chemical equation is a balanced account of a chemical transaction.

Boyle’s Law and Charle’s
Law Boyle’s Law
• Relation between pressure (p) and volume (V)
• Statement − At constant temperature, the pressure of a fixed amount (number of moles, n) of a gas is inversely proportional to its volume.
• Explanation − Based on kinetic theory:
• Number of particles and their average kinetic energy is constant for a given mass of gas.
• When volume of a certain mass of gas is reduced to half, the particles have lesser space to move around.
• The number of collision of the particles with the walls of the container doubles, thus increasing the pressure to twice the original value.
• Mathematically,

• From the above equation, it is found that at constant temperature, the product of pressure and volume of a fixed amount of a gas is constant.
• The value of K1 depends upon
• amount of the gas
• temperature of the gas
• units of p and V
Graphical representation of Boyle’s law

• Each line is called isotherm (at constant temperature plot).
• If at constant temperature,
V1 = Volume of a gas at pressure p1
V2 = Volume of the same gas at pressure p2
Then,
p1V1 = p2V2 = Constant

• Relationship between density (d) and pressure (p):
We know that,

Where, m = Mass of a gas
V = Volume of the gas

• From the above equation, it is known that density is proportional to the pressure of a fixed amount of a gas.
• Significance of Boyle’s law:
• Mountaineers carry oxygen cylinders with them as at higher altitudes as the pressure is low.
Example
Rita has two cylinders. One is empty and the other contains compressed nitrogen at 25 atm. She wants to distribute the gas in the two cylinders. To do so, she connects the two cylinders. If the volume of the cylinder containing the gas is 50 L and that of the empty one is 80 L, then what will be the pressure inside the two cylinders?
Solution:
According to Boyle’s law,
P1V1 = P2V2
Given, p1 = 25 atm
V1 = 50 L
V2 = (50 + 80) L = 130 L
Now, 25 atm × 50 L = p2 × 130 L

Hence, the pressure inside the cylinders is 9.62 atm.
Charles’ Law
• Relation between temperature (T) and volume (V)
• Statement − At constant pressure, the volume of a fixed amount of a gas is directly proportional to its absolute temperature.
• Explanation − On the basis of kinetic theory
• Average kinetic energy of the particles of a gas is directly proportional to the absolute temperature of the gas
• When temperature is increased at constant pressure, the kinetic energy of the particles increases.
• The number and intensity of collisions with the walls of the container increase, thereby increasing the volume at constant pressure.
• Mathematically,
V ∝ T
⇒ V = K2T where K2 = Proportionality constant
• The value of K2 depends upon
• pressure of the gas
• amount of the gas
• unit of volume
• Graphical representation
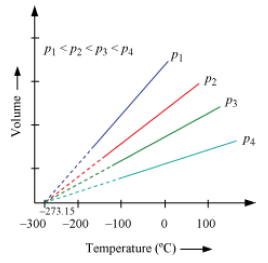
• Straight line
• Interception on zero volume at 273.15°C
• Each line is called isobar (constant pressure plot).
• Derivation
For each degree rise in temperature, volume of a gas increases by 1 / 273.15 of the original volume of the gas at 0°C.
Suppose, V0 = Volume of a gas at 0°C
Vt = Volume of the same gas at t°C
Then,

According to Kelvin temperature scale (also called absolute temperature scale or thermodynamic scale),
T = 273.15 + t
T0 = 273.15
From equation (i), we obtain

Or, we can write

• Significance of Charle’s law:
• Hot air is filled in the balloons used for meteorological purposes.
Example
It is desired to increase the volume of 5 L of a gas by 40% without changing the pressure. To what temperature should the gas be heated if its initial temperature is 298 K?
Solution:
Desired increase in the volume of gas = 40% of 5 L

= 2 L
Therefore, final volume of the gas = (5 + 2 ) L = 7 L
Applying Charles’ law,

Now, V1 = 5 L
T1 = 298 K
V2 = 7 L

Standard Temperature and Pressure(STP)
The pressure and temperature of the gas keeps varying frequently. Hence, we choose a standard value for temperature and pressure to which the gas volumes can be referred. The standard value chosen are 0°0°C or 273K for temperature and 1 atm or 760 mm of Hg for pressure and are commonly known as S.T.P.
Diffusion
Diffusion is defined as the random movement of gaseous molecules from regions of higher concentration to regions of lower concentration. It is a physical process and can only occur if the gases do not react with each other.
Graham’s Law of Diffusion:
It states that the rate of diffusion of gas is inversely proportional to the square root of its density at the given temperature and pressure.

It means that the rate of diffusion is inversely proportional to the square root of mass of the gas.
The Mole Concept
The Mole Concept: A Brief Overview
Mole defines the quantity of a substance. One mole of any substance will always contain 6.022 × 1023 particles, no matter what that substance is.
Therefore, we can say:
• 1 mole of sodium atoms (Na) contains 6.022 × 1023 sodium atoms.
• 1 mole of sodium ions (Na+) contains 6.022 × 1023 sodium ions.
• 1 mole of hydrogen atoms (H) contains 6.022 × 1023 hydrogen atoms.
• 1 mole of hydrogen molecules (H2) contains 6.022 × 1023 hydrogen molecules.
The word ‘mole’ is derived from the Latin word ‘moles’ which means ‘heap’ or ‘pile’. It was first used by the German chemist Wilhelm Ostwald in 1896. It was accepted universally much later, in 1967, as a way of indicating the number of atoms or molecules in a sample.
Thus, mole can be defined as a unit of measurement used for determining the number of atoms or molecules or ions in a given sample. It is also used to express the number of reactants and products in a chemical reaction.
The Mole Concept: A Brief Overview
Consider the formation of water by the combination of hydrogen and oxygen.
2H2 + O2 → 2H2O
This reaction implies that 2 moles of hydrogen molecules combine with 1 mole oxygen molecules to form 2 moles of water molecules.
When carbon (C) reacts with oxygen (O), carbon dioxide is produced. Can you write the chemical equation for the same?
The chemical equation for the reaction is:

In this reaction, one atom of carbon combines with one molecule (or two atoms) of oxygen to form one molecule of carbon dioxide. We can also say that in this chemical reaction, 12 u of carbon combines with 32 u of oxygen to give 44 u of carbon dioxide. Clearly, we can represent the quantities of substances in terms of their masses.
However, a chemical equation only indicates the numbers of atoms or molecules taking part in the chemical reaction. Therefore, it is easier to represent the quantities of substances involved in a chemical reaction by the numbers of their atoms or molecules rather than their masses. In order to do the same, the concept of mole is used.
Mole Concept
In 1909, the French physicist Jean Perrin found that one gram atom of any element contains the same number of atoms and one gram molecule of any substance contains the same number of molecules, which is equal to 6.022 × 1023.
He proposed naming this number in honour of the Italian physicist Amedeo Avogadro. Hence, 6.022 × 1023 is known as Avogadro’s number (or Avogadro’s constant) and the amount of a substance containing 6.022 × 1023 atoms /molecules/ions is called a mole.
Mole is a counting unit in chemistry as it is used to express large numbers of atoms or molecules. One mole of any substance can be defined as the amount of a substance that contains as many particles (atoms, molecules or ions) as there are atoms in 12 g of carbon-12 isotope. So,
1 mole of oxygen atoms (O) = 6.022 × 1023 oxygen atoms
1 mole of oxygen molecules (O2) = 6.022 × 1023 oxygen molecules
Know Your Scientist

Jean Perrin (1870-1942) was a French physicist. He was awarded the Nobel Prize in Physics in 1962, for his contribution to the establishment of the atomic nature of matter, while conducting research on Brownian motion. In 1895, he showed that cathode rays are made up of negatively charged particles. He is also known for explaining the origin of solar energy through thermonuclear reaction of hydrogen (nuclear fusion) in the sun.
In 1908, he studied Brownian motion using an ultramicroscope and gave experimental confirmation to the hypothesis that the random motion of suspended particles is due to the particulate nature of matter and the inter-particle interactions. He is also credited with estimating the size of a water molecule and the number of molecules of water present in a given amount of water.

Amedeo Avogadro (1776-1856) was an Italian lawyer; however, his interest in the natural sciences led him to study physics and mathematics privately. In 1809, while teaching the natural sciences in Vercelli, he hypothesized that under the same conditions of temperature and pressure, equal volumes of gases contain the same number of particles. This hypothesis later came to be known as Avogadro’s law.
Mole Concept
The molar mass of a substance can be defined as the mass of one mole of a substance in grams. It is numerically equal to atomic/molecular/formula unit mass in u.

The mass of one atom is called atomic mass and its unit is unified mass (u), while the mass of one mole of atoms is called molar mass of atoms and its unit is gram (g). Molar mass of atoms is also called gram atomic mass.
For example, the atomic mass of nitrogen (N) is 14 u, while its gram atomic mass is 14 g. So, while 14 u of nitrogen contains only 1 atom of nitrogen, 14 g of nitrogen contains 1 mole of nitrogen atoms, i.e., 6.022 × 1023 nitrogen atoms.
The mass of one molecule is called molecular mass and its unit is unified mass (u), while the mass of one mole of molecules is called molecular mass and its unit is gram (g). When molecular mass is expressed in grams, it is called gram molecular mass or gram molecule.
For example, the molecular mass of oxygen (O2) is 32 u, while its gram molecular mass is 32 g. So, while 32 u of oxygen contains only 1 molecule of oxygen, 32 g of oxygen contains 1 mole of oxygen molecules, i.e., 6.022 × 1023 oxygen molecules.
The volume of one mole of any substance is called its molar volume.
The molar volume of a gas at STP is numerically equal to 22.4 L.
Mole Concept
Solved Examples
Easy
Example 1:
Calculate the mass of 3.3 moles of ammonia molecule.
Solution:
Molar mass of ammonia molecule (NH3) = 17 g
Number of moles of ammonia molecule = 3.3
We know that:

So,

Medium
Example 2:
Calculate the volume of 14 g of nitrogen gas at STP.
Solution:
Mass of nitrogen gas (N2) = 14 g
Molar mass of nitrogen gas = 28 g
We know that:

The volume of 1 mole of a gas at STP is 22.4 L.
Therefore,

Avogadro’s Law
In 1811, Avogadro hypothesized that under the same conditions of temperature and pressure, equal volumes of all gases contain an equal number of moles. For example, at the same temperature and pressure, the two gases, oxygen and nitrogen possessing the same volume contain the same number of molecules. This hypothesis is named Avogadro’s law. The mole concept provides the following information.
• If one mole of a substance (atoms, molecules or ions) is present, then the number of elementary particles present in that substance is equal to 6.022 × 1023.
• The mass of one mole of a substance (atoms, molecules or ions) is equal to its molar mass.
• While carrying out reactions, scientists require the number of atoms and molecules. This requirement is fulfilled by the use of the mole concept as follows:
1 mole = 6.022 × 1023 = Relative mass in grams.
Avogadro’s Law
Relationship between Mole, Avogadro’s Number and Mass
The relationship between mole, Avogadro’s number and mass is summarized in the given figure.
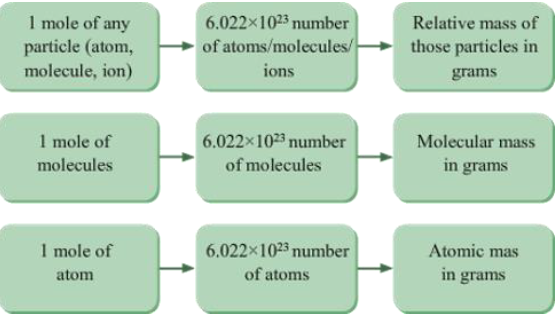
Applications of Avogadro’s Law
• It provides an explanation for Gay-Lussac’s law.
The volumes of different combining gases bear a simple ratio to one another because according to Avogadro’s law at constant temperature and pressure equal volumes of gases contain the same number of molecules.
• It helps in determination of atomicity of gases.
Consider the formation of hydrogen chloride gas by the direct combination of hydrogen and chlorine gases:
H2 + Cl2 → 2 HCl
According to Avogadro’s law:
1 molecule of H2 + 1 molecule of Cl2 → 2 molecules of HCl
Or, 1/2 molecule of H2 + 1/2 molecule of Cl2 → 1 molecule of HCl
As atoms are indivisible, therefore, half a molecule of H2 and Cl2 indicate they both contain two atoms per molecule.
• It helps in determination of molecular formula of a gas.
Consider the formation of hydrogen chloride gas by the direct combination of hydrogen and chlorine gases:
H2 + Cl2 → 2 HCl
According to Avogadro’s law:
1 molecule of H2 + 1 molecule of Cl2 → 2 molecules of HCl
Or, 1/2 molecule of H2 + 1/2 molecule of Cl2 → 1 molecule of HCl
Or, 1 atom of H + 1 atom of Cl → 1 molecule of HCl
Hydrogen chloride gas has one atom of hydrogen and one atom of chlorine. Therefore, its molecular formula is HCl.
• It helps in the establishment of the relationship between molecular mass and vapour density (VD).

• It provides the relation between gram molecular mass and gram molecular volume.
Molar volume of a gas = 22.4 L
Gram molecular mass of a gas occupies 22.4 L and contains 6.02 × 1023 molecules/atoms of the gas.
Gay-Lussac’s Law
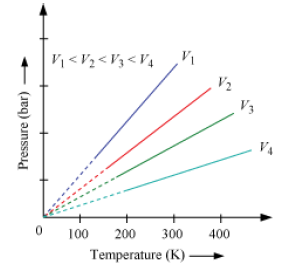
This law gives the relationship between pressure and temperature. According to this law, at constant volume, the pressure of a fixed amount of a gas is directly proportional to the temperature. It can be represented mathematically as, Mathematically,

If at constant volume,
p1 = Pressure of a gas at T1
p2 = Pressure of the same gas at T2
Then,
Mole Concept and Molar Masses
• 1 mole of any substance can be defined as:
• Amount of a substance that contains as many particles (atoms, molecules or ions) as there are atoms in 12 g of the 12C isotope
• Avogadro number or Avogadro constant (NA); it is equal to 6.022 × 1023 particles
• Example − 1 mole of oxygen atoms = 6.022 × 1023 atoms of oxygen 1 mole of carbon dioxide molecules = 6.022 × 1023 molecules of carbon dioxide 1 mole of sodium chloride = 6.022 × 1023 formula units of sodium chloride
• Relative atomic mass: Relative atomic mass of an element is the ratio of the average mass of one atom of an element to one-twelfth of the mass of an atom of carbon-12.

Molar mass of a substance can be defined as:
• Mass of one mole of a substance in grams
• Numerically equal to atomic/molecular/formula mass in u.
• Example − Molar mass of CO2 = 44.011 g mol−1
• Relative molecular mass: It is defined as the ratio of the mass of a molecule to the atomic mass unit of the molecule. It is a unitless quantity.
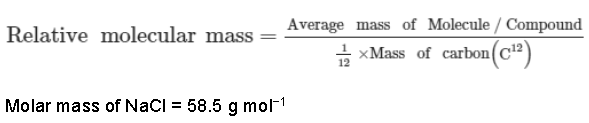
Examples
1. What number of moles contains 3.011 × 1023 molecules of glucose?
Solution:
1 mole of glucose is equivalent to 6.022 × 1023 molecules of glucose.
Hence, 3.011 × 1023 molecules of glucose will be present in

Thus, 0.5 mole of glucose contains 3.011 × 1023 molecules of glucose.
2. What is the mass of a fluorine molecule?
Solution:
1 mole of fluorine molecule contains 6.022 × 1023 molecules and weighs 38 g.

Atomicity
• It is defined as the total number of atoms of constituent elements which combine to form a molecule.
• One molecule of hydrogen combines with one molecule of chlorine to form two molecules of hydrogen chloride.
• One molecule of hydrogen or chlorine contains two atoms of each.
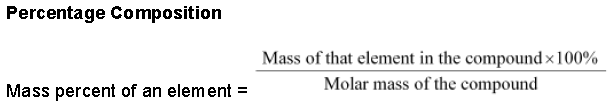
Example
What is the mass percent of oxygen in potassium nitrate? (Atomic mass of K = 39.10 u, atomic mass of N = 14.007 u, atomic mass of O = 16.00 u)
Solution:
Atomic mass of K = 39.10 u (Given)
Atomic mass of N = 14.007 u (Given)
Atomic mass of O = 16.00 u (Given)
Therefore, molar mass of potassium nitrate (KNO3)
= 39.10 + 14.007 + 3(16.00)
= 101.107 g
Therefore, mass percent of oxygen in KNO3

• Empirical formula and molecular formula:

• Empirical formula is determined if mass % of various elements are known.
• Molecular formula is determined from empirical formula if molar mass is known.
Example
A compound contains 92.26% carbon and 7.74% hydrogen. If the molar mass of the compound is 26.038 g mol−1, then what are its empirical and molecular formulae?
Solution:
Mass percent of carbon (C) = 92.26% (Given)
Mass percent of hydrogen (H) = 7.74% (Given)
Therefore, 100 g of the compound contains 92.26 g and 7.74 g of of hydrogen
Number of moles of carbon present in the compound =92.26 / 12.011 = 7.68 mol
Number of moles of hydrogen present in the compound = 7.74 / 1.008 =7.68 mol
Thus, in the given compound, carbon and hydrogen are present in the ratio C : H = 7.68 : 7.68 = 1 : 1
Therefore, the empirical formula of the compound is CH.
Empirical formula mass of CH = (12.011 + 1.008)g = 13.019 g
Molar mass of the compound = 26.038 g (Given)
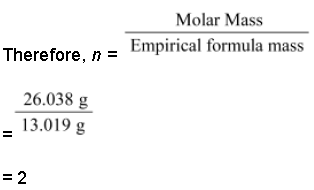
Hence, the molecular mass of the compound is (CH)n, i.e., (CH)2 or C2H2.
Interconversion Among Number of Moles, Mass and Number of Molecules

Stoichiometric Calculations in Balanced Chemical Equations
• An example of a balanced chemical equation is given below.

From the above balanced chemical equation, the following information is obtained:
• One mole of C3H8(g) reacts with five moles of O2(g) to give three moles of CO2(g) and four moles of H2O(l).
• One molecule of C3H8(g) reacts with five molecules of O2(g) to give three molecules of CO2(g) and four molecules of H2O(l).
• 44 g of C3H8(g) reacts with (5 × 32 = 160) g of O2(g) to give (3 × 44 = 132) g of CO2(g) and (4 × 18 = 72) g of H2O(l).
• 22.4 L of C3H8(g) reacts with (5 × 22.4 = 112) L of O2(g) to give (3 × 22.4 = 67.2) L of CO2(g) and (4 × 22.4 = 89.6) L of H2O(l).
Example
Nitric acid (HNO3) is commercially manufactured by reacting nitrogen dioxide (NO2) with water (H2O). The balanced chemical equation is represented as follows:
3NO2(g) + H2O(I) → 2HNO 3(aq) + NO(g)
Calculate the mass of NO2 required for producing 5 moles of HNO3.
Solution:
According to the given balanced chemical equation, 3 moles of NO2 will produce 2 moles of HNO3.
Therefore, 2 moles of HNO3 require 3 moles of NO2.
Hence, 5 moles of HNO3 require =2/3X5 moles of NO2
= 7.5 moles of NO2
Molar mass of NO2 = (14 + 2 × 16) g mol-1
= 46 g mol-1
Thus, required mass of NO2 = (7.5 × 46) g
= 345 g
• Limiting reagent or limiting reactant:
• Reactant which gets completely consumed when a reaction goes to completion
• So called because its concentration limits the amount of the product formed
Example
Lead nitrate reacts with sodium iodide to give lead iodide and sodium nitrate in the following manner:
Pb(NO3)2 + 2NaI → PbI2 + 2NaNO3
What amount of sodium nitrate is obtained when 30 g of lead nitrate reacts with 30 g of sodium iodide?
Solution:
Molar mass of Pb(NO3)2 = 207 + [{14 + (16X3)}X2]
= 331 g mol-1
Molar mass of NaI = (23 + 127) = 150 g mol-1
According to the given equation, 1 mole of Pb(NO3)2 reacts with 2 moles of NaI, i.e.
331 g of Pb(NO3)2 reacts with 300 g of NaI to give PbI2 and NaNO3
Thus, 30 g of Pb(NO3)2 will react with (30 × 300) / 331 g of NaI = 27.19g of NaI However, we have 30 g of NaI. So, NaI is present in excess and Pb(NO3)2 is the limiting reagent.
Now, number of moles in 30 g of Pb(NO3)2 30 g = 331/30 = 0.09 mole
According to the equation, 1 mole of Pb(NO3)2 gives 2 moles of NaNO3.
So 0.09 moles of Pb(NO3)2 will give (2 × 0.09) moles of NaNO3 = 0.18 moles of NaNO3
• Reactions in solutions:
Ways for expressing the concentration of a solution −
• Mass per cent or weight per cent (w/w%)
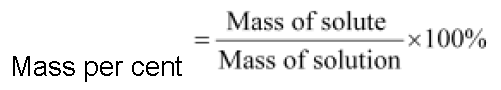
Example
4.4 g of oxalic acid is dissolved in 200 mL of a solution. What is the mass per cent of oxalic acid in the solution? (Density of the solution = 1.1 g mL−1)
Solution:
Density of the solution = 1.1 g mL−1
So the mass of the solution = (200 mL) × (1.1 g mL−1)
= 220 g
Mass of oxalic acid = 4.4 g
Therefore, mass per cent of oxalic acid in the solution

• Mole fraction:
If a substance ‘A’ dissolves in a substance ‘B’, then mole fraction of

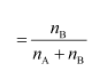
nA − Number of moles of A
nB − Number of moles of B
Example
A solution is prepared by dissolving 45 g of a substance X (molar mass = 25 g mol−1) in 235 g of a substance Y (molar mass = 18 g mol−1). Calculate the mole fractions of X and Y.
Solution:

And, mole fraction of Y, nY = 1 − nX
= 1 − 0.121
= 0.879
• Molarity:
Number of moles of solute in 1 L of solution

For a given solution, the molarity equation is as follows:
M1V1 = M2V2
M1 = Molarity of a solution when its volume is V1
M2 = Molarity of the same solution when its volume is V2
Examples
1. 10g of HCl is dissolved in enough water to form 500 mL of the solution. Calculate the molarity of the solution.
Solution:
Molar mass of HCl = 36.5 g mol−1

= 0.548 M
2. Commercially available concentrated HCl contains 38% HCl by mass. What volume of concentrated HCl is required to make 2.5 L of 0.2 M HCl? (Density of the solution = 1.19 g mL−1)
Solution:
38% HCl by mass means that 38g of HCl is present in 100 g of the solution.
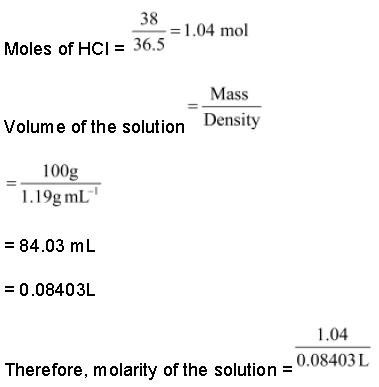
= 12.38 M
According to molarity equation,
M1V1 = M2V2
Here,
M1 = 12.38 M
M2 = 0.2 M
V2 = 2.5 L
Now, M1V1 = M2V2
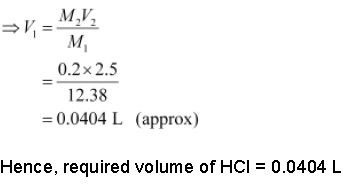
• Molality:
Number of moles of solute present in 1 kg of solvent
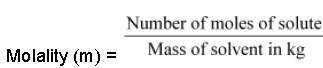
Example
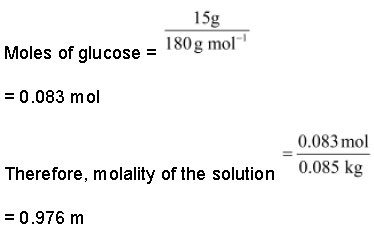
What is the molality of a solution of glucose in water, which is labelled as 15% (w/w)?
Solution:
15% (w/w) solution means that 15 g of glucose is present in 100 g of the solution, i.e. (100 − 15) g = 85 g of water = 0.085 kg of water