ICSE Class 10 students can refer to ICSE Class 10 Organic Chemistry Notes given below which have been prepared as per the latest syllabus and guidelines issued by ICSE Board. All chapter wise revision notes for ICSE Class 10 Chemistry have been prepared by teachers have strong understanding of Chemistry. Read the notes prior to the exams to get better marks in exams
Organic Chemistry ICSE Class 10 Chemistry Revision Notes
Students can refer to the quick revision notes prepared for Chapter Organic Chemistry in Class 10 ICSE. These notes will be really helpful for the students giving the Chemistry exam in ICSE Class 10. Our teachers have prepared these concept notes based on the latest ICSE syllabus and ICSE books issued for the current academic year.
Revision Notes ICSE Class 10 Chemistry Organic Chemistry
Please refer to the detailed notes below
Organic Chemistry
It is the chemistry of specific carbon compounds except oxides, carbonates and carbides.
Hydrocarbons
Organic compounds composed of carbon and hydrogen only.
Examples: Methane (CH4), ethane (C2H6)
Unique Nature of Carbon
Tetravalency of Carbon
• Carbon forms four covalent bonds by mutually sharing its four electrons with other atoms.
• It is hence tetravalent or exhibits tetravalency.
Catenation
• It is the tendency of an element to form chains of identical atoms.
• Catenation is maximum in carbon because the value of the C–C bond energy is maximum.
• Carbon undergoes self-linking forming straight, branched and closed chains.

• Catenation and tetravalency also result in the formation of single, double and triple bonds.
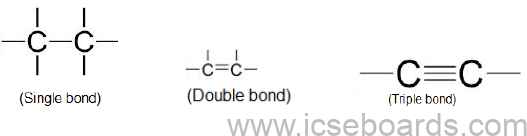
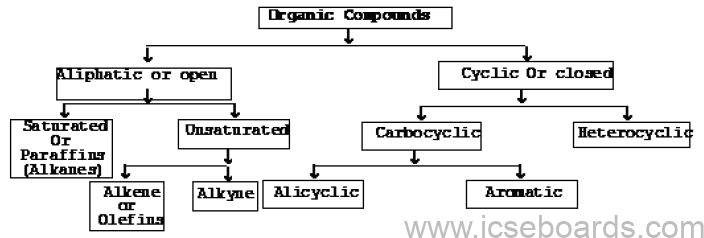
Classification of Organic Compounds
Homologous Series
It is a group of organic compounds with a similar structure and similar chemical properties in which the
successive compounds differ by a CH2 group.
Characteristics of a homologous series
i. Each member of the series differs from the preceding one by the addition of a CH2 group and by 14
amu.
ii. All members of a homologous series share a general formula.
For example, the general formula for alkane is CnH2n+2 and that for alkene is CnH2n.
iii. The physical properties of the members show gradation in properties as molecular mass increases.
iv. The chemical properties also show gradient similarity.
For example, methane and ethane react with chlorine to form methyl chloride and ethyl chloride,
respectively.
CH4 + Cl2 → CH3Cl
C2H6 + Cl2 → C2H5Cl
v. All members of a homologous series can be prepared by the same general method of preparation.
For example, alcohols can be prepared from alkyl halides.

Significance of a Homologous Series
i. Helps in the systematic study of organic compounds.
ii. Predicts the properties and the nature of other elements of the series if the same is known of the first
few members.
Isomers
Compounds with the same molecular formula but different structural formula are known as isomers, and
the phenomenon is known as isomerism.
Examples: Butane and isobutane are two different compounds with the same molecular formula C4H10.
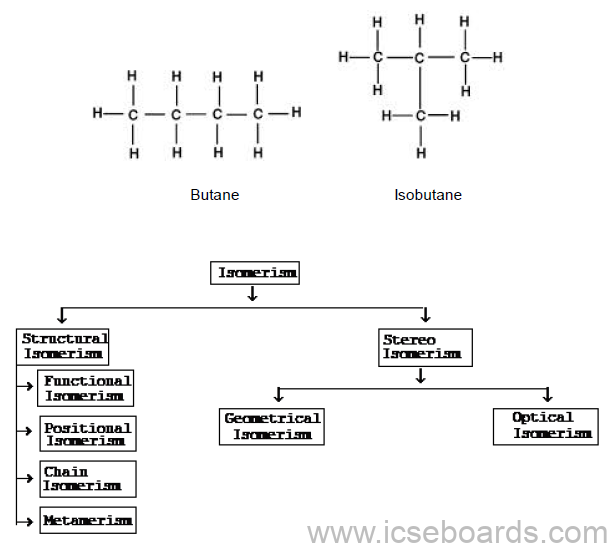
Causes of Isomerism
i. Difference in the mode of linking of atoms.
ii. Difference in the arrangement of atoms or groups in space.
Different Types of Structural Isomerism
i. Chain isomerism
Two or more compounds which have a similar molecular formula but different arrangement of
carbon atoms in straight or branched chains are referred to as chain isomers, and the
phenomenon is known as chain isomerism.
ii. Position isomerism
When two or more compounds with the same molecular formula differ in the position of the
substituent atom or functional group on the carbon atom, they are called position isomers, and the
phenomenon is known as position isomerism.
iii. Functional isomerism
Two or more compounds with the same molecular formula but different functional groups are called
functional isomers, and the phenomenon is known as functional isomerism.
iv. Metamerism
It arises because of unequal distribution of alkyl groups on either side of the functional groups in
the molecules.
Nomenclature
It is the system of assigning names to organic compounds.
The Systems of Nomenclature Are
i. Trivial system
ii. IUPAC (International Union of Pure and Applied Chemistry) system
According to the IUPAC system, the name of an organic compound consists of three parts:
i. Root word
ii. Suffix
iii. Prefix
i. Root word
It depends on the number of carbon atoms present in the longest carbon chain selected.
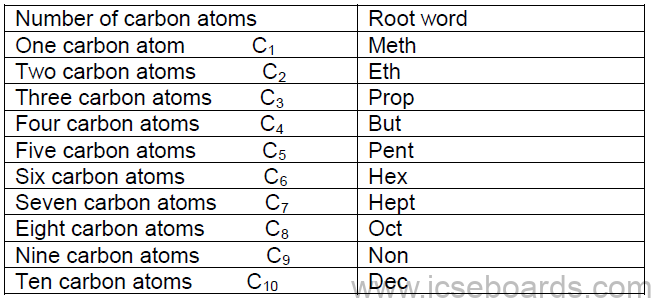
ii. Suffix
The root word is followed by an appropriate suffix, which represents the nature of the bond in a
carbon–carbon atom.

iii. Prefix
It denotes the substituent, alkyl or functional group and its position in the carbon chain.
Di-, tri- and tetra- are used for two, three and four groups of the same type, respectively.
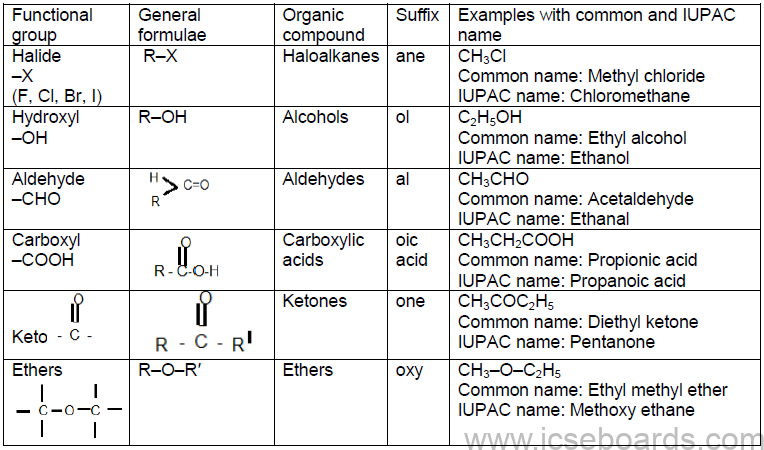
Functional Group
It is an atom or a group of atoms which defines the structure (or the properties of a particular family) of
organic compounds.
Characteristics of a Functional Group
i. Compounds of the same functional group are identified using the same types of tests.
ii. The physical and chemical properties of the compounds of different functional groups are different.
iii. There exists a homologous series of compounds containing a particular type of functional group.
Alkanes
• Alkanes are hydrocarbons in which all the linkages between the carbon atoms are single covalent
bonds.
• Compounds are known as saturated hydrocarbons because all the four valencies of carbon are fully
satisfied.
• General formula : CnH2n+2
• These hydrocarbons are relatively unreactive under ordinary conditions so they are also called
paraffins.
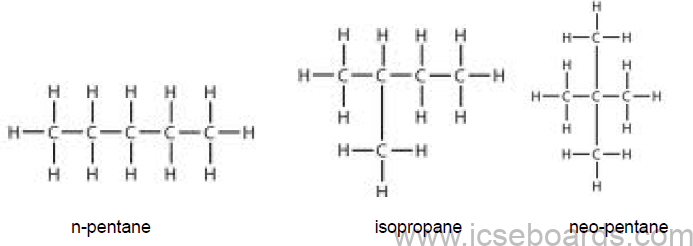
Isomerism in Alkanes
• Alkanes with more than three carbon atoms form isomers.
• The various isomers differ in the framework of the carbon chains.
Example:
Isomers of Pentane (C5H12)
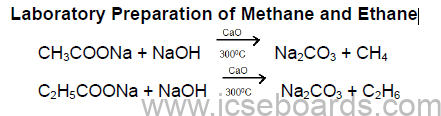
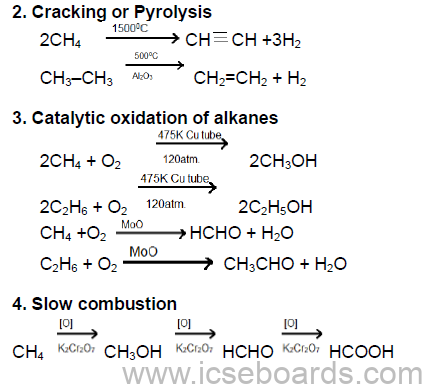
Methods of Preparation of Methane and Ethane
1. From iodomethane or bromoethane:
CH3I + 2[H] → CH4 + HI
C2H5I + 2[H] → C2H6 + HI
2. Methane is produced on addition of water to aluminium carbide at room temperature.
Al4C3 + 12H2O → 3CH4 + 4Al (OH)3
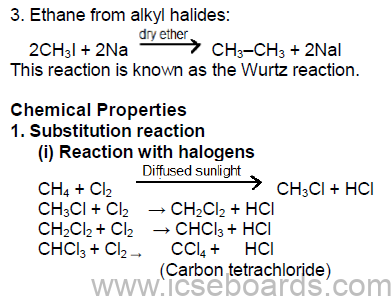
(ii) Reaction with oxygen
CH4 + 2O2 →CO2 + 2H2O
2C2H6 + 7O2 → 4CO2 + 6H2O
Insufficient supply of air
2CH4 + 3O2 → 2CO + 4H2O
2C2H6 + 5O2 → 4CO + 6H2O
Alkenes
• Alkenes are unsaturated aliphatic hydrocarbons containing a carbon–carbon double bond.
• They are also called olefins because of their tendency to form oily products.
• The general formula of alkenes is CnH2n.
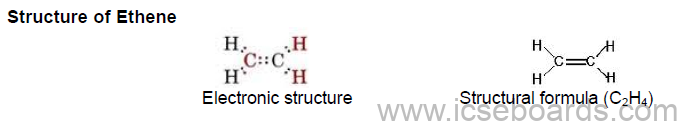
• Two carbon atoms linked by a double covalent bond.
• A double covalent bond is formed by sharing of two pairs of electrons between the two carbon atoms.
• Four C–H single covalent bonds and one C=C double covalent bond.
• It is a planar molecule and all bond angles (H–C–H and H–C=C) are of 120°.
Preparation of Ethene
i. Dehydration of ethyl alcohol

Chemical properties
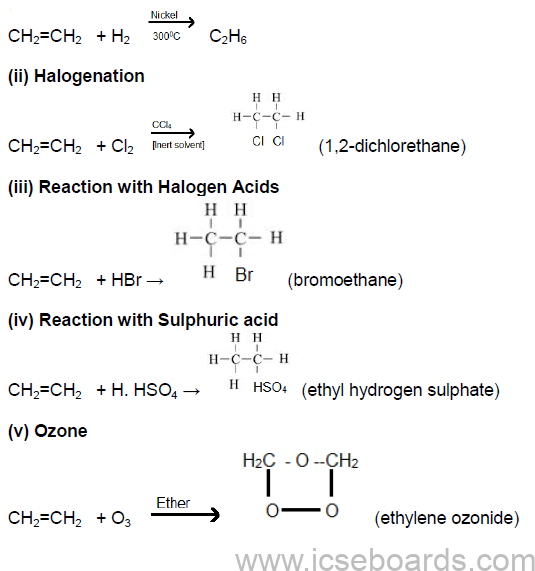
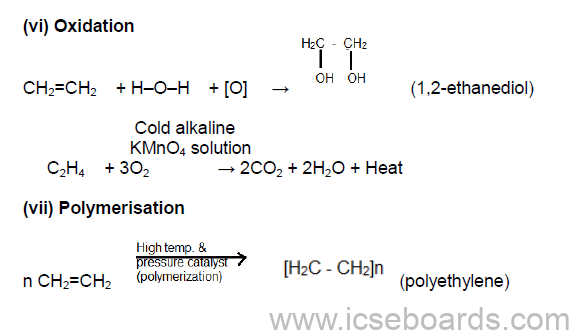
1. Addition Reactions
(i) Catalytic hydrogenation
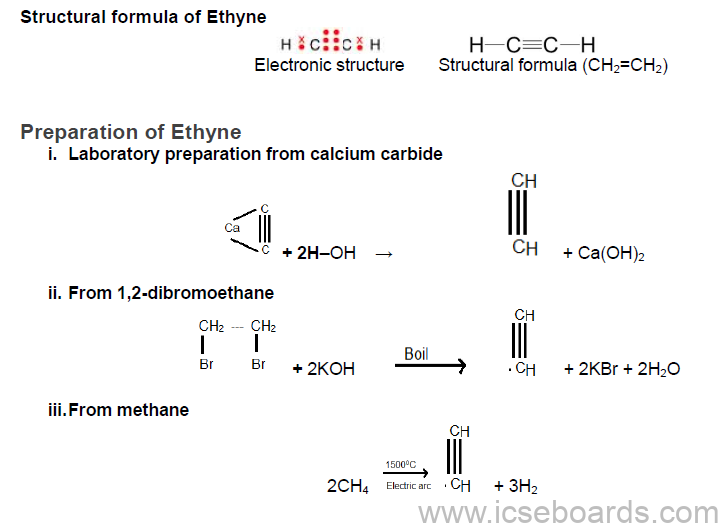
Alkynes
• Alkynes are unsaturated aliphatic hydrocarbons containing a carbon–carbon triple bond in their
molecule.
• The general formula of alkynes is CnH2n−2.
• They are more reactive than alkenes because of the presence of a triple bond, often referred to as an
acetylenic linkage.
Chemical Properties
1. Addition Reactions

Alcohols
• Alcohols are hydroxyl derivatives of alkanes obtained by replacement of one, two or three hydrogen
atoms of alkanes by the corresponding number of –OH groups.
• The hydroxyl group is the functional group of alcohols.
• The general molecular formula of alcohols is CnH2n+1 OH.
Preparation of Ethanol
(i) Laboratory preparation by hydrolysis of alkyl halides


Carboxylic Acids
• Carboxylic acids are organic compounds containing a carboxylic group (–COOH) attached to an alkyl
group or to a hydrogen atom.
• Representation of carboxylic acids: R-COOH (R is either –H or alkyl)
• The functional group of carboxylic acids: –COOH (carboxylic)
• The acidic character in carboxylic acids is because of the presence of the replaceable hydrogen atom
in the carboxylic group.
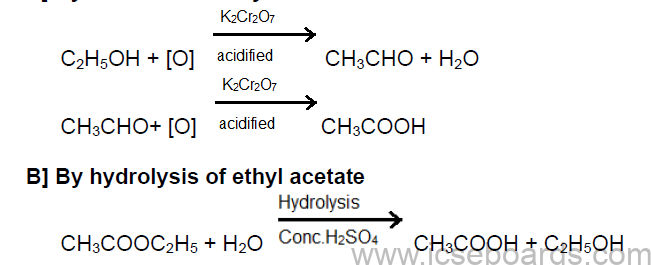
Preparation of Acetic Acid
A] By oxidation of ethyl alcohol
Chemical Properties
1. It is a weak acid and turns blue litmus red.
2. Reaction with Alkalis
CH3COOH + NaOH → CH3COONa + H2O
CH3COOH + NH4OH → CH3COONH4 + H2O
3. Reaction with Carbonates
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3→ CH3COONa + H2O + CO2
4. Reaction with Alcohols

5. Reaction with PCl3
CH3COOH + PCl5 → CH3COCl + POCl3 + HCl
6. Reduction
CH3COOH + 4[H] →C2H5OH + H2O



