Students can refer to the following Sample Paper ICSE Class 10 Chemistry Set I with Answers provided below based on the latest syllabus and examination guidelines issued for ICSE Chemistry. All specimen papers have been prepared covering all chapters given in ICSE Chemistry book for Class 10. You should also refer to ICSE Class 10 Chemistry Solutions.
Sample Paper ICSE Class 10 Chemistry Set I with Answers
Subject: Chemistry
Answer to this paper must be written on the paper provided separately.
You will not be allowed to write during the first 15 minutes.
This time is to be spent in reading the question paper.
The time given at the head of this Paper is the time allowed for writing the answers.
Section I is compulsory. Attempt any four questions from Section II.
The intended marks for the questions or parts of questions are given in brackets [ ].
Sample Paper ICSE Class 10 Chemistry Set I
SECTION – I [40 MARKS]
Attempt all questions.
Question 1
a) Choose the correct answer from the options given below: (5)
i. A weak electrolyte from the following is:
A. Acetic acid
B. Oxalic acid
C. Ammonium hydroxide
D. Sodium hydroxide
ii. Electron affinity is maximum in:
A. Alkali metals
B. Alkaline earth metals
C. Halogens
D. Inert gases
iii. The main components duralumin is:
A. Aluminium, Magnizium and Manganese
B. Alluminium, Manganese
C. Copper, Zinc and Tin
D. Copper, Alluminium, Tin
iv. The drying agent used to dry NH3 is:
A. P2O5
B. conc. H2SO4
C. CaCl2
D. CaO
v. The general formula of alkene is:
A. CnH2n-2
B. CnH2n+2
C. CnH2n
D. CnH2n+3
b) Write balanced chemical equations for each of the following: (5)
i. Catalytic oxidation of ammonia
ii. Action of concentrated nitric acid on Sulphur
iii. Action of concentrated sodium hydroxide on Zinc oxide
iv. Reaction between acetic acid with ethanol in the presence of concentrated Sulphuric acid.
v. Action of dilute hydrochloric acid on iron.
c) State any one observation for each of the following: (5)
i. Dilute Hydrochloric acid is added to Silver Nitrate solution.
ii. Concentrated Nitric acid is added to Copper turnings.
iii. Mixture of Ammonium Chloride and Sodium Hydroxide is heated.
iv. Ammonium hydroxide solution is added in excess to copper sulphate solution.
v. NaOH solution is added to calcium nitrate solution.
d) Rewrite the following by inserting appropriate word/words: (5)
i. Magnesium Nitride reacts with water to liberate Ammonia.
ii. Lead bromide conducts electricity.
iii. Starch iodide paper turns blue black in the presence of Chlorine.
iv. Hydrogen chloride molecule contains a covalent bond.
v. Acid salts are formed by replacement of the ionisable hydrogen ions of the acid by a metallic ion or ammonium ion.
e) i. Given: CH4 + 2O2 → CO2 + 2H2O (5) If 16grms of methane is burned find the mass of CO2 produced?
ii. Find the number of moles and molecules present in 7.1 g of C12 (At. Wt. Cl = 35.5)
iii. Calculate the vapour density of ethene (C = 12, H = 1)
f) Identify the terms: (5)
i. The energy required to remove an electron from valance shell of a neutral isolated gaseous atom.
ii. Crushing of the ore into a fine powder.
iii. The property by which carbon bonds with itself to form a long chain.
iv. A bond formed by a shared pair of electrons with both electrons coming from the same atom.
v. A substance that conducts electricity in molten or aqueous state.
g) Arrange the following as per the instruction given in the brackets. (5)
i. Li, F, N (Increasing order of electronegativity)
ii. Na, Al, Cl (Increasing order of Ionization potential)
iii. O2, N2, Cl2 (Increasing order of number of covalent bonds)
iv. Ar, He, Ne (Order of preference of discharge at the cathode)
v. Br, F, Cl (Decreasing order of atomic radius)
h) i. Draw the structural formula for each of the following: (5)
1. But-l-ene
2. Propanoic acid
3. di – Ethyle Ether
ii. Draw the structural isomers of C2H4.
Sample Paper ICSE Class 10 Chemistry Set I
SECTION – II [40 MARKS]
Answer any four questions.
Question 2
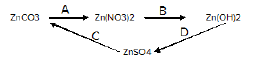
a) Write balanced equations for following conversions:
b) Show the formation of H3O + using the electron dot diagram. State the types of bonds present in it.(3)
c) Distinguish between the following pairs of compounds using the test given within the brackets.(3)
i. Calcium sulphite and calcium carbonate (using dil. HCl)
ii. Calcium nitrate and potassium nitrate (using a flame test)
iii. Lead nitrate solution and Zinc nitrate solution (using an alkali)
Question 3
a) Study the table and answer the following questions:
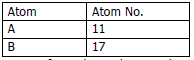
i. Compare the positions of A and B in the Periodic Table.
ii. Which is more metallic?
iii. Write equations for the formation of ions of A and B.
iv. What type of bond is formed between A and B? Mention its physical state and solubility in water.
b) Identify the gas evolved in each of the following cases: (4)
i. Con. Sulphuric acid added to copper.
ii. Water is added to calcium carbide.
iii. Dilute hydrochloric acid is added to Zinc sulphide.
iv. Con. Nitric acid added to copper.
Question 4
a) Write a balanced equation for the following: (4)
i. C2H5Br + alcoholic KOH →
ii. CaC2 + 2H2O →
iii. C2H4 + Br2 →
iv. C2H5OH + Na →
b) State how the following conversions can be carried out: (3)
i. Ethyl chloride to ethyl alcohol
ii. Ethyl alcohol to ethene
iii. Ethyl bromide to ethanol
c) Give the correct IUPAC name for each of the compounds whose structural formula are given below.

Question 5
a) i. Name the chief ore of Aluminum and the process of concentration of the ore.
ii. Write the balanced equations for the conversions of the above ore of aluminum to pure alumina.
iii. Name one alloy of Aluminum. (6)
b) A compound gave a following data: (4)
C = 57.82%, O = 38.58% and the rest hydrogen. Its relative molecular mass is 166. Find its empirical formula and molecular formula. (C = 12, O = 16, H = 1)
Question 6
a) i. Copy and complete the following table:

ii. Name the catalyst used in the catalytic oxidation of ammonia.
b) Give appropriate scientific reasons for each of the following statements. (2)
i. Electrolysis of molten lead bromide is considered to be a redox reaction.
ii. Although copper is a good conductor of electricity it is a non-electrolyte.
c) Mention the property of conc. H2SO4 exhibited in each of the following reaction with: (2)
i. sugar
ii. metallic chloride
iii. non-metal such as carbon
d) Test to distinguish dil. H2SO4 from dil HCI and dil. HNO3 (2)
Question 7
a) Answer the following questions pertaining to laboratory preparation of Hydrogen chloride: (4)
i. Write an equation for the laboratory preparation of Hydrogen Chloride.
ii. Name the drying agent used.
iii. Name the method of collecting Hydrogen Chloride gas.
iv. Condition required for the preparation of HCI gas.
b) Give reasons for each of the following: (3)
i. Direct absorption of HCI gas in water is not preferred.
ii. All glass apparatus is used in the laboratory preparation of HNO3.
iii. NaCl has a high melting point.
c) Give one point of difference between the following pairs of terms given: (3)
i. Calcination and Roasting.
ii. Polar and Non-Polar covalent compounds.
iii. Strong electrolyte and weak electrolyte.