EXERCISE-12A
Question 1. Name the three constituent of an atom and state their mass and charge of each. How are they distributed in an atom?
Solution:
Three constituents of an atom are:
Electrons have a mass of 9.1 X10-31 kg, -1.6 X 10-19C charge
Neutrons have a mass of 1.6749 X10-27 kg, the charge is zero.
Protons have a mass of 1.6726 X 10-27 kg, the charge is +1.6 X 10-19 C
Question 2. Define the term:
(i) Atomic number
(ii) Mass number.
Solution:
(i) Atomic number – The term “atomic number” refers to the quantity of protons in the nucleus.
(ii) Mass number- The mass number refers to the total number of nucleons in the nucleus.
Question 3. What is nucleus of an atom? Compare its size with that of the atom. Name its constituents. How the number of these constituents is determined by the atomic number its atomic model.
Solution:
The nucleus in the center of an atom 10-5 to 10-4 larger than an atom, measuring in the range of 10-15 m to 10-14 m it is made up of neutrons and protons. An atom has Z number of electrons, Z number of protons, and A – Z number of neutrons if Z is its atomic number and A is its mass number. The atom is denoted by the sign A/Z x where x is the chemical symbol for the element.
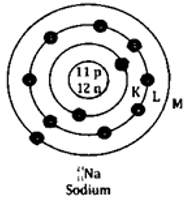
Question 4. State the atomic number and mass number of 23/11 Na and draw its atomic model.
Solution:
It is given that,
Atomic number Z = 11
Mass number A = 23
Number of neutrons A – Z = 12
Question 5. What are isotopes? Give one example.
Solution:
Isotopes: Atoms of the same element that have different mass numbers A but the same atomic number Z are referred to as isotopes. Example: Hydrogen has three isotopes

Protium, Deuterium, Tritium.
Question 6. What are isobars? Give one example.
Solution:
Isobars are atoms from several elements that have the same mass number A but a different atomic number Z. Example: 1123na & 1223mg
Question 7. Name the atoms of a substance having same atomic number, but different mass numbers. Give one example of such a substance. How do the structures of such atoms differ?
Solution:
Isotopes are atoms of a material with the same atomic number but differing masses.
Example: Hydrogen has three isotopes

Structure of each isotope differs by the number of neutrons in its nuclei.
Question 8. What is radioactivity? Name two radioactive substances.
Solution:
Nuclear phenomena include radioactivity. It is the process of atoms’ nuclei spontaneously emitting radiations, such as or and, when they decay. Example: uranium, radium.
Question 9. A radioactive substance is oxidized. What changes would you expect to take place in the nature of radioactivity? Explain your answer.
Solution:
The characteristics of radiation won’t alter. This is due to the nuclear nature of radiation.
Question 10. A radioactive source emits three types of radiations.
(i) Name the three radiations.
(ii) Name the radiations which are deflected by the electric field.
(iii) Name the radiation which is most penetrating.
(iv) Name the radiation which travels with the speed of light.
(v) Name the radiation which has the highest ionizing power.
(vi) Name the radiation consisting of the same kind of particles as the cathode rays.
Solution:
(i) Three types of radiations: Alpha, beta and gamma.
(ii) Alpha and beta radiations
(iii) Gamma radiations
(iv) Gamma radiations
(v) Alpha radiations
(vi) Beta radiations
Question 11. A radioactive source emits three types of radiations.
(a) Which radiation has zero mass?
(b) Name the radiation which has the lowest ionizing power.
(c) Name the radiation which has the lowest penetrating power.
(d) Give the charge and mass of particles composing the radiation in part (c).
(e) When the particle referred to in part (c) becomes neutral, they are found to be the atoms of rare gas. Name this rare gas and draw a model of its neutral atom.
(f) From which part of the atom do these radiations come?
Solution:
(a) Gamma radiations have zero mass.
(b) Gamma radiations have the lowest ionizing power.
(c) Alpha particles have lowest penetrating power.
(d) Alpha particle has positive charge equal to 3.2 x 10-19C and rest mass equal to 4 times the mass of proton i.e. 6.68 x 10-27 kg.
(e) The gas is Helium.

Question 12. The diagram in figure shows a radioactive source S placed in a thick lead walled container. The radiations given off are allowed to pass through a magnetic field. The magnetic field (shown as x) acts perpendicular to the plane of paper inwards. Arrows shows the paths of the radiation A, B and C.

(a) Name the radiations labeled A, B and C.
(b) Explain clearly how you used the diagram to arrive at the answer in part(a).
Solution:
Radiations labeled A, B and C are γ , α and β respectively. Radiation is gamma radiation because it has no charge and is thus undeflected by a magnetic field. Because of its enormous mass and a lower likelihood of being deflected than beta radiation, radiation B is alpha radiation. The Fleming left-hand rule indicates the deflection’s direction. Since alpha and beta radiation have opposing charges, their deflection directions are also different.
Question 13. In the following Figure shows a mixed source R of alpha and beta particles in a thick lead-walled container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by x.
(a) Show in the diagram how the particles get affected.
(b) Name the law used in part (a)

Solution:
(a)

(b) Fleming’s left hand rule
Question 14. In following Figure shows a radioactive source S in a thick lead walled container having a narrow opening. The radiations pass through an electric field between the plates A and B.

(a) Complete the diagram to show the paths of α , β and γ radiations.
(b) Why is the source S kept in a thick lead walled container with a narrow opening?
(c) Name the radiation which is unaffected by the electrostatic field.
(d) Which radiation is defleced the most? Given reason
(e) Which among the three radiations causes the least biological damage?
Solution:
(a)

(b) The radioactive materials are stored in heavy lead canisters with a very small aperture to prevent radiations from escaping in other directions, which might harm living tissue.
(c) Gamma radiations.
(d) Beta radiations.
(e) Alpha particle.
Question 15. Explain why alpha and beta particles are deflected in an electric or a magnetic field, but gamma rays are not deflected in such a field.
Solution:
α and β In an electric or magnetic field, and are deflected because they are positive and negative charged particles, respectively, whereas radiations are not charged particles and do not deflect.
Question 16. Is it possible to deflect γ – radiations in a way similar to α and β -particles, using the electric or magnetic field? Give reasons.
Solution:
Gamma radiation is neutral and does not deflect under the influence of an electric or magnetic field, hence it is not feasible to deflect it in the same manner as alpha and beta particles using these fields.
Question 17. State following four properties each of α , β and γ radiations: (a) Nature, (b) Charge, (c) Mass and (d) Effect of electric field.
Solution:

Question 18. Arrange α, β, or γ radiations in ascending order of their (i) ionising powers, and (ii) penetrating powers.
Solution:
Gamma radiation has the least ionising power, whereas beta particles have less ionising power—100 times that of gamma radiation—and alpha radiation has the most ionising strength, or 10,000 times that of gamma radiation. Gamma radiation has the greatest penetration power whereas alpha particles have the least.
Question 19. State the speed of each of α, β and γ – radiations.
Solution:
Speed of α radiation is nearly 107 m/s.
Speed of β radiation is about 90% of the speed of light or 2.7 x 108 m/s.
Speed of γ radiation is 3 x 108 m/s in vacuum.
Question 20. (i) What is the composition of α, β and γ -radiations?
(ii) Which one has the least penetrating power?
Solution:
(i) Two protons and two neutrons make up alpha radiation. A beta particle is an electron that moves quickly. Gamma radiations are electromagnetic waves, similar to x-rays, or photons.
(ii) The weakest piercing radiation is alpha radiation.
Question 21. How γ – radiations are produced? Mention two common properties of gamma radiations and visible light.
Solution:
When a nucleus is in an excited state, gamma radiations are generated (i.e., it has an excess of energy). Gamma radiation is produced as a result of the excess energy. The electric and magnetic fields do not deflect gamma radiation like they do light. The speed of gamma radiation is equal to the speed of light.
Question 22. An α-particle captures (i) one electron, (ii) two electrons.
In each case, what does it change to?
Solution:
(i) It will change into singly charged helium I He+
(ii) Neutral helium atom
Question 23. ‘Radioactivity is a nuclear phenomenon’. Comment on this statement.
Solution:
The rate of decay of the radioactive substance is not affected by any physical changes (such as changes in pressure and temperature) or chemical changes (such as severe heating, freezing, action of strong electric and magnetic fields, chemical treatment, oxidation, etc.). This demonstrates unequivocally that the cause of radioactivity cannot be the orbital electrons, which are susceptible to such alterations. Therefore, the nucleus should possess the radioactivity. Radiation is a nuclear phenomenon
Question 24. What kind of change takes place in a nucleus when a β – particle is emitted? Express it by an equation. State whether
(a) Atomic number and
(b) Mass number are conserved in a radioactive β – decay?
Solution:
The number of nucleons in the nucleus (protons and neutrons) remains constant when a particle is emitted, however the number of neutrons decreases by one and the number of protons increases by one.
The change may be depicted as follows if a radioactive nucleus P with mass number A and atomic number Z emits a beta particle to create a daughter nucleus Q with mass number A and atomic number Z+1:
ZAP β particle → z+qAQ+-10e
(a) The ‘Z’ atom’s number is not preserved. It is raised by one
(b) A conserves its mass number.
Question 25. A certain radioactive nucleus emits a particle that leaves its mass unchanged, but increased its atomic number by one. Identify the particle and write its symbol.
Solution:
Beta particle
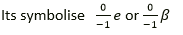
Question 26. What happens to the (i) atomic number, (ii) mass number of an element when (a) α – particle (b) β -particle and (c) γ -radiation are emitted?
Sol:
(a) Atomic number falls by two.
(b) Atomic number rises by one.
(c) Atomic number remains unchanged.
Question 27. What happens to the position of an element in the periodic table when it emits an alpha particle?
Solution:
The daughter element moves two spaces to the left of the parent element in the periodic table after generating an alpha particle.
Reason: If -decay results in a parent nucleus X into a new daughter nucleus Y, then -decay may be represented as:

(b) The daughter element moves one position to the right of the parent element in the periodic table after emitting a -particle.
Reason: If – decay results in a parent nucleus X into a new daughter nucleus Y, then -decay may be represented as

The resultant nucleus therefore has an atomic number of Z. As a result, it is located in the same spot in the periodic table as the parent element.
Question 28. What changes occurs in the nucleus of radioactive elements when it emits (a) an alpha particle (b) beta particle and (c) gamma radiations? Give one example in each case in support of your answer.
Solution:
The following changes occur when an atom emits
An alpha particle: atomic number falls by 2 and mass number falls by 4.
Example: 92238U + 90234Th + 24he
A beta particle: atomic number up by one, but mass number does not change.
Example: 614C + 714N + -10β
Gamma particle: it does not change anything in the nucleus, the energy of the nucleus falls number does not change.
Example: ZAX → ZAX + γ
Question 29. (a)An atomic nucleus A is composed of 84 protons and 128 neutrons. The nucleus A emits α -particle and is transformed into a nucleus B. What is the composition of B?
(b) The nucleus B emits a β -particle and is transformed into a nucleus C. What is the composition of C?
(c) What is the mass number of nucleus A?
(d) Does the composition of nucleus C change if it emits a γ -radiation?
Solution:
(a) The 82 protons and 126 neutrons that make up the element B
(b) The 83 protons and 125 neutrons that make up the element C.
(c) The mass number of nucleus A = no. of protons + no. of neurons = 84 + 128=212.
(d) The composition of the nucleus C won’t change.
Question 30. A certain nucleus A (mass number 238 and atomic number 92) is radioactive and becomes a nucleus B (mass number 234 and atomic number 90) by the loss of one particle.
(i) What particle was emitted?
(ii) Explain how you are arrived at your answer.
(iii) State the change in the form of a reaction.
Solution:
(i) The alpha particle was released
(ii) This is due to a 2 and a 4 drop in the atomic and mass numbers, respectively.
(iii) 92238P → 90234Q + 24α
Q31. State whether the following nuclear disintegration are allowed or not (star indicate an exited state). Give reason if it is not allowed.
(i) ZAX → ZAX + γ
(ii) ZAX → Z-2AX + 24He
Solution:
(i) This is acceptable.
(ii) Because mass number is not preserved, this is not permitted.
Question 32. A nucleus is 1124Na is β-radioactive.
(i) What are the numbers 24 and 11 called?
(ii) Write the equation represents β-decay.
(iii) What general name is given to the product nucleus with respect to 1124Na
Solution:
(i) An atom is specified by the symbol ZAX where X is the chemical symbol for the element.
Z is the atomic number and A is the mass number of an atom, then the atom contains Z number of electrons.
24 is the mass number and 11 is the atomic number.
(ii) 1124Na → 1224Mg + -10e
(iii) Isobar
Question 33. A nucleus of stable phosphorus has 15 protons and 16 neutrons.
(i) What is its atomic number and mass number?
(ii) The nucleus of radio phosphorous has one neutron more than the stable nucleus. What will be its atomic number and mass number?
(iii) What will be the atomic number and mass number of new nucleus formed by decay of a β-particle by the radio phosphorus in part(b)?Solution:
(i) An atom has Z number of electrons, Z number of protons, and A – Z number of neutrons if Z is its atomic number and A is its mass number. The atom is denoted by the symbolZAX where X is the element’s chemical symbol. The mass number is 31, while the atomic number is 15.
(ii) An atom has Z number of electrons, Z number of protons, and A – Z number of neutrons if Z is its atomic number and A is its mass number. The atom is identified by the symbolZAX where X is the element’s chemical symbol. The mass number is 32 and the atomic number is 15.
(iii) An atom has Z number of electrons, Z number of protons, and A – Z number of neutrons if Z is its atomic number and A is its mass number. The atom is denoted by the symbolZAX where X is the element’s chemical symbol. The mass number is 32 while the atomic number is 16.
Question 34. An element P disintegrates by α-emission and the new element suffers two further disintegrations, both by β -emission, to form an element Q. Explain the fact that P and Q are the isotopes.
Solution:
Following the emission of one alpha and two beta particles, the atomic number of Q formed is the same as that of P. This is because the atomic number of P decreases by 2 and its mass number decreases by 4 as a result of the emission of one alpha particle, and then increases by 1 as a result of the emission of each beta particle. P and Q are the isotopes as a result.
Question 35. A nucleus ZAX emits 2 α particles and 1 β particles to form a nucleus 85222R222R. Find the atomic nucleus and mass number of X.
Solution:
After emitting two alpha particles
Atomic number = Z – 4
Mass number = A – 8
After emitting a beta particle
Atomic number = Z – 4 + 1
Mass number = A – 8
Final atomic number = 85
85 = Z – 4 + 1
Z = 88
Final mass number 222
222 = A – 8
A = 230
Question 36. Complete the following sentence:
(i) The mass number (A) of an element is not changed when it emits ______________.
(ii) The atomic number of a radioactive element is not changed when it emits…………
(iii) During the emission of a beta particle, the ________ number remains same.
Solution:
(i) The mass number (A) of an element is not changed when it emits β and γ radiations.
(ii) The atomic number of a radioactive element is not changed when it emits γ radiations.
(iii) During the emission of a beta particle, the mass number remains same.
Question 37. Complete the following nuclear change :
(i) zaP → Q + -10β
(ii) 92238U → 90234Th + ⋯…… + Energy
(iii) 92238P ∝ → Qβ → Rβ → S
(iv) ZAX ∝ → X1 γ → X2 2β → X3
(v) X β → X1 α → X2 α → 69172X3
Solution:
(i) x + 1aQ
(ii) 92238U → 90234Th + 24He + Energy
(iii) 90234Q, 91234R, 92234S
(iv) 90234X1, 91234X2, zA-4X3
(v) 71176X2 73180X1, 72180X
Question 38. What are radio isotopes? Give one example of a radio isotope. State one use of radio isotopes
Solution:
Radio isotopes: The isotopes of some elements with atomic number Z. Example: carbon (Z=6, A=14).
Radio isotopes are used in medical and scientific and industrial fields. Radio isotopes such as 92 232U are used as fuel for atomic energy reactors
Question 39. Why are the alpha particles not used in radio therapy?
Solution:
Due to the fact that they cannot pierce human skin
Question 40. Why do we usually use isotopes emitting gamma radiations as radioactive tracers in medical science?
Solution:
Gamma radiation may readily travel through the human body due to its extremely high penetration strength. As a result, they are employed in medical science as radioactive tracers.
Question 41. When does the nucleus of an atom become radioactive?
Solution:
Nuclei become unstable or radioactive when the number of neutrons in them is much more than the number of protons.
Question 42. Which of the following is the radioisotope in pair?
(i) 612C, 614C
(ii) 1530P, 1532P
(iii) 1930K, 1940K
Give a reason for your answer.
Solution:
(i) 614C The explanation is that there are more neutrons than protons.
(ii) 1532P The reason is that there are more neutrons than protons.
(iii) 1940K The explanation is that there are more neutrons than protons.
Question 43. State the medical use of radioactivity.
Solution:
Radiation treatment is used to treat a wide variety of disorders, including leukemia, cancer, etc. Cobalt-60 radiation is used to treat cancer by destroying the cells in the patient’s malignant tumor.
For diagnostics, salts containing weak radioactive isotopes such as radio-sodium chloride, radio-iron, and radio-iodine are utilized. These radioactive isotopes are known as tracers.
Question 44. Arrange α, β, and γ radiation in ascending order of their biological damage. Give reason.
Solution:
a<β<γ An -particle’s penetrating capability is relatively low since it rapidly loses energy as as it passes through a medium. It only has an air penetration range of 3 to 8 cm. A thin card sheet or a thick piece of paper can stop it with ease.
The -particles have greater penetrating power than the -particles. They can go through thin card sheets, thin sheets of aluminum foil, and nearly 5 meters of air, but a 5 mm thick aluminum sheet will stop them.
While -rays have a strong penetrating power. It is approximately 104 times that of α-particles and 102 times greater than that of – and -particles, respectively. They can squeeze through a sheet of iron that is 30 cm thick or 500 meters of air. To stop them, a thick covering of lead is needed.
Question 45. Name two main sources of nuclear radiation. How are these radiations harmful?
Solution:
Two main sources of nuclear radiation are:
1. Fallout from nuclear power plants and other sources that is radioactive.
2. Nuclear waste disposal.
These radiations are dangerous because they damage human living tissues and result in radiation burns when they contact the body.
Question 46. State two safety measures to be taken while establishing a nuclear power plant.
Solution:
The following safety measures must be taken in a nuclear power plant:
1. Lead and steel walls must surround the nuclear reactor to prevent radiation from leaking out into the environment when it is in regular operation.
2. The nuclear reactor needs to be housed in an earthquake-, fire-, and explosion-resistant airtight building made of sturdy concrete.
3. The reactor core needs a backup cooling system so that, if one system fails, the other cooling system can step in and prevent the core from melting due to overheating.
Question 47. (i) What is meant by nuclear waste?
(ii) Suggest one effective way for the safe disposal of nuclear waste.
Solution:
(i) The radioactive substance eventually transforms into the lead after disintegrating, although it retains some radioactivity. We refer to this as nuclear waste.
(ii) The most efficient approach for safely disposing of nuclear waste is the delay and decay method.
Question 48. State three safety precautions that you would take while handling the radioactive substances.
Solution:
(i) To assist reduce exposure, those handling radioactive materials should utilize mechanical tools such tongs, clamps, tweezers, etc.
(ii) When handling radioactive materials, workers should don protective gear including lead-lined lab coats, gloves, and safety glasses. They should also take this gear off before leaving the lab or work area.
(iii) To prevent radiation from escaping in other directions, all radioactive items should be kept in specially built thick lead containers with a very tight entrance.
Question 49. Why should a radioactive substance not be touched by hand?
Solution:
Because radioactive materials emit damaging radiation, touching them with bare hands can lead to radiation burns and the death of living tissues in the body.
Question 50. What do you mean by background radiations? Name its sources. Is it possible for us to keep ourselves away from it?
Solution:
Background radiation is the name given to low-temperature microwave radiation that comes from all directions in space and strikes the surface of the earth.
Sources of background radiation include
(a) Sunlight radiation.
(b) Earth’s rocks that have traces of radioactive materials.
(c) Isotopes are present in nature.
(d) Synthetic radioactive elements.
No, we are unable to protect ourselves from these radiations.
EXERCISE-12A
MULTIPLE CHOICE TYPES
Question 1. A radioactive substance emits radiations:
a) α, β, and γ simultaneously
b) In the order α, β, and γ one by one
c) X-ray and Y-ray
d) α or β
Solution: d) α or β
Question 2. In β- emission from a radioactive substance, an electron is ejected. This electron comes from:
a) The outermost orbit of an atom
b) The inner orbits of an atom
c) The surface of substance
d) The nucleus of an atom
Solution: d) The nucleus of an atom
Question 3. The least penetrating radiation is:
a) α – particles
b) β – particles
c) x – rays
d) γ – radiations
Solution: a) α – particles
Question 4. The radiation suffering the maximum deflection in a magnetic field is:
a) α – particles
b) β – particles
c) X – rays
d) γ – radiations
Solution: b) β – Particles
EXERCISE-12B
Question 1. What do you mean by nuclear energy? What is responsible for its release?
Solution:
Nuclear energy is the energy generated when an atom’s nuclei combine or when an unstable radioactive nucleus decays during a nuclear process, such as during fusion or fission.
Question 2. Write down the Einstein’s mass-energy equivalence relation, explaining the meaning of each symbol used in it.
Solution:
E = mc2 is Einstein’s formula for the mass-energy equivalency.
Where c is the speed of light and E is the energy released as a result of the mass loss, where E is the energy released due to the loss in the mass Δm and c is the speed of light.
Question 3. (i) What is a.m.u? Express 1 a.m.u. in MeV.
(ii) Write the approximate mass of a proton, neutron and electron in a.m.u.
Solution:
(i) The atomic mass unit is used to represent the mass of atomic particles (a.m.u.). 931 MeV of energy is equal to 1 a.m.u. of mass.
(ii) Mass of proton = 1.00727 a.m.u.
Mass of neutron = 1.00865 a.m.u.
Mass of electron = 0.00055 a.m.u.
Question 4. What is nuclear fission? Name the particle used for it. Write one fission reaction.
Solution:
When a heavy nucleus is bombarded with slow neutrons, it breaks into two virtually identical light nuclei, a process known as nuclear fission.
92235U + 01N → (92236U) → 56144Ba + 3689Kr + 301 n + Energy
Question 5. Name two isotopes of uranium.
Which of the isotope mentioned in part (a) above is easily fissionable? Give reason.
State whether the neutron needed for fission reaction of the isotope mentioned in part (b) above, is slow or fast?
Solution:
92235U and 92235U Experimental research shows that the isotope of 92235Uis more easily fissionable because it can fission with a slow neutron, as opposed to 92235U which can only fission with a fast neutron.
Question 6. Write the approximate value of the energy released in the fission of one nucleus of 92235U what is the reason for it?
Solution:
When one nucleus of 92235U splits apart, an initial 190 MeV of energy is released this energy was released owing to a loss in mass, where the total of the masses of the product nuclei is smaller than the sum of the masses of the parent nucleus and neutron.
Question 7. Complete the following nuclear fission reaction.
(i) 92235U + 01N → 56Ba + 92Kr + 301 n + ⋯
(ii) 92235U + 01N → 148La + 3585Br + ⋯ + 01N + Energy
Solution:
(i) 92235U + 01N → 56Ba + 92Kr + 301 n + Energy
(ii) 92235U + 01N → 148La + 3585Br + 301 n + 01N + Energy
Question 8. What do you mean by the chain reaction in nuclear fission? How is it controlled?
Solution:
A large quantity of energy is released during a chain reaction, which is a sequence of nuclear fissions in which the neutrons created during each fission trigger other fissions. It is regulated by using moderators like graphite, heavy water, etc. to absorb part of the neutrons released during the fission process. The energy produced from fission may then be used for constructive purposes.
Question 9. State two uses of nuclear fission
Solution:
(i) A nuclear bomb uses it.
(ii) A nuclear reactor, which is used to produce electricity, uses it to release energy at a slow, regulated rate.
Question 10. Give two differences between the radioactive decay and nuclear fission.
Solution:

Question 11. (i) what is nuclear fission? Give one example and write its nuclear reaction.
(ii) What other name is given to nuclear fusion? Give reason.
Solution:
(i) Nuclear fission is the splitting of a heavy nucleus into two nearly identical light nuclei by the bombardment of slow neutrons.
When neutrons strike uranium with a Z = 92, it divides into two pieces called barium (Z = 56) and krypton (Z = 36), and a significant quantity of energy is produced that appears as a result of the decrease in the mass.
92235U + 01N → (92236U) → 56144Ba+3689Kr+301n + Energy
(ii) Thermonuclear reaction is another name for nuclear fusion. This is due to the high temperature at which nuclear fusion occurs.
Question 12. Why is a very high temperature required for the process of nuclear fusion? State the approximate temperature required.
Solution:
Due to their positive charges, two nuclei repel one another so strongly when they come close to one another that they do not fuse. As a result, nuclear fusion is impossible at normal pressure and temperature. Hence, a high temperature of around 107 K and a high pressure are needed to make fusion feasible. Both nuclei gain enough kinetic energy at such a high temperature as a result of thermal agitation to overcome the force of repulsion between them as they approach one another, causing the nuclei to fuse.
Question 13. (a) Write one nuclear fusion reaction.
(b) State the approximate value of energy released in the reaction mentioned in part (a).
(c) Give reason for the release of energy stated in part (b).
Solution:

(c) In all three deuterium nuclei fuse to form a helium nucleus with a release of 21•6 MeV energy. When two deuterium nuclei 12H fuse, nucleus of helium isotope 23He. He is formed and 3•3 MeV energy is released. This helium isotope again gets fused with one deuterium nucleus to form a helium nucleus 24HeHe and 18•3 MeV of energy is released in this process.
Question 14 Complete the following fusion reaction:
(i) 23He + 12H→2He + 1H+Energy
(ii) 12H + 12H→2He + 1H+ Energy
Solution:
(i) 23He +12H→24He +1 H + Energy
(ii) 12H + 12H → 23He +1H + Energy
Question 15. Name the process, nuclear fission or nuclear fusion,
(i) In which the energy released per unit mass is more?
(ii) Which is possible at ordinary temperature?
Solution:
(i) Nuclear fusion energy released more mass in a unit.
(ii) Nuclear fission is possible at ordinary temperature.
Question 16. (i) State the similarity in the process of nuclear fission or fusion
(ii) State two differences between the process of nuclear fission or fusion.
Solution:
(i) Neutrons and a significant quantity of energy are released during both fission and fusion.
(ii) Nuclear fission: When exposed to neutrons, a heavy nucleus divides into two almost identical light pieces. Nuclear fusion is achievable at very typical pressure and temperature: A heavy nucleus is created when two light nuclei interact under conditions of extreme pressure and heat. Possible only at very high pressures and temperatures (≈107 K)
Question 17. Give two examples of nuclear fusion.
Solution:
(i) 11H+11H→12H+0.42MeV
(ii) 12H+12H→13He+11H+4.0MeV
Question 18. What is the source of energy of Sun or stars?
Solution:
The source of energy in the Sun and stars is the nucleus fusion of light nuclei such as hydrogen present in them in their inner part. This takes place at a very high temperature and high pressure due to which helium nucleus is formed with the release of high amount of energy.
Question 19. Name the following nuclear reaction:
(i) 92234U + 01n→3890Sr + 54143Xe + 301 n+γ
(ii) 13H + 12H→ 24He + 01n + γ
Solution:
(i) Nuclear fission
(ii) Nuclear fission
EXERCISE-12B
MULTIPLE CHOICE TYPES
Question 1. The particle used in nuclear fission for bombardment is :
a) Alpha particle
b) Proton
c) Beta particle
d) neutron
Solution: d) Neutron
Question 2. The temperature required for the process of nuclear fusion is nearly:
a) 1000 K
b) 104K
c) 105K
d) 107K
Solution: d) 107K
EXERCISE-12B
Question 1. In fission of one uranium – 235 nucleuses, the loss in mass is 0.2 a.m.u. Calculate the energy released.
Solution:
It is given that,
1a.m.u. = 1.66 × 10-27 kg
0.2 a.m.u. = 0.2 × 1.66 × 10-27 kg
Δm=0.332 Δ10-27 kg
E = 0.332 × 10-27 kg × (3 × 108)2
E = 2.988 × 10-11 J

Question 2. When four hydrogen nuclei combine to form a helium nucleus in the interior of sun, the loss in mass is 0.0265 a.m.u. How much energy is released?
Solution:
It is given that,
Δm = 0.0265 a.m.u.
1 a.m.u. liberates 931.5 MeV of energy.
Thus, energy liberated equivalent to 0.0265 a.m.u.
= 0.0265 a,m,u, x 931MeV
= 24.7 meV

