Students of ICSE Class 10 should refer to Hydrogen Chloride ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Hydrogen Chloride
Hydrogen Chloride is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Hydrogen Chloride ICSE Class 10 Chemistry Questions
Hydrogen Chloride ICSE Class 10 Chemistry Questions
Introduction
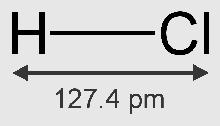
• Molecular Formula of hydrogen chloride – HCl.
• Molecular mass of HCl = 1 + 35.5 = 36.5
• Vapour density = ½ mol. wt. = ½ × 36.5 = 18.25
• Hydrogen Chloride – Common Name : Spirit of salt
• Hydrochloric acid – Common Name : Muriatic acid
• HCl is a polar covalent compound.
A. Occurrence
1. Where is hydrogen chloride found in the free state?
Ans. In gastric juices of the stomach (mammals) and in volcanic eruptions.
2. HCl is a polar covalent compound.
Ans. HCl is a polar covalent compound due to the difference in the electronegativities of H and Cl. Chlorine is more electronegative thus attracting shared pair of electrons more towards itself.
B. Preparation of Hydrogen Chloride Gas
General Methods of Preparation of Hydrogen Chloride
3. (a) Give the balanced chemical equation for preparation of hydrogen chloride by synthesis or direct combination.
Ans.

(b) Give the conditions required to carry out the reaction.
Ans. The reaction is carried out in the presence of diffused sunlight and moisture acting as catalyst.
(c) Why can’t the above reaction be carried out in dark or direct sunlight.
Ans. The reaction does not take place in dark, whereas in direct sunlight the reaction is explosive.
4. Answer the following questions on the basis of laboratory preparation of hydrogen chloride gas.
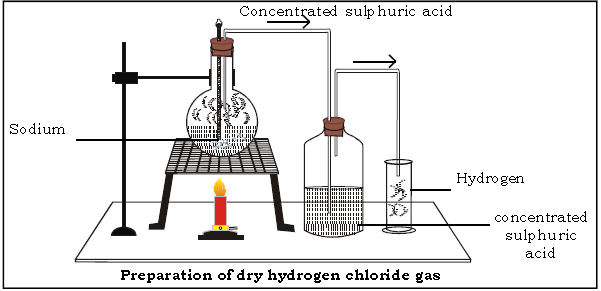
(a) Name the reactants used.
Ans. Sodium chloride and conc. sulphuric acid.
(b) Give the balanced chemical equation for the preparation of hydrochloric acid in the laboratory.
Ans.
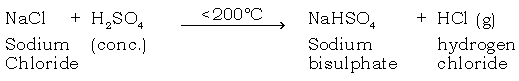
(c) Why is the temp maintained below 200ºC?
Ans. (1) Above 200ºC, NaCl combines with H2SO4 to form sodium sulphate. This is an undesirable compound, which forms a hard crust that is difficult to remove.
(2) Fuel is wasted as it is a bad conductor of heat.
(3) Glass apparatus may crack.
(d) Why are the reactants mentioned preferred?
Ans. NaCl is cheap and easily available and H2SO4 is a non-volatite acid, hence these reactants are preferred.
(e) Why is nitric acid not used in the preparation?
Ans. (i) Nitric acid is a volatile acid.
(ii) It has a low B.P. and will volatalize off along with the fumes of hydrogen chloride.
(iii) It cannot displace another volatile acid from its compound.
(f) Name the drying agent for hydrogen chloride.
Ans. Conc. Sulphuric acid.
(g) Why are other drying agents not used?
Ans. Other drying agents would react with HCl and hence are not used to purify HCl
(h) Why CaO or P2O5 cannot be used to dry HCl gas. Explain using chemical equations.
Ans. (i) CaO is basic, it combines with the acidic hydrogen chloride to
form salt and water.

(i) Name the method of collection.
Ans. HCl gas is collected by upward displacement of air.
(j) Why is the above method of collection used? Or Why is HCl collected by upward displacement of air?
Ans. HCl gas is collected by upward displacement of air because:-
(i) It is heavier than air
(ii) It is highly soluble in water and hence cannot be collected over water.
(k) Compare the density of HCl gas with air and state the solubility of HCl gas in water.
Ans. HCl gas is 1.28 times heavier than air. HCl is highly soluble in water, 1 volume of water dissolves 452 volumes of hydrogen chloride at ordinary temperature.
(l) How would you determine whether the gas jar is completely filled with hydrogen chloride gas?
Ans. A glass rod dipped in ammonia is brought near the mouth of the gas jar, if dense white fumes are observed we can conclude that the jar is filled with HCl.
C. Properties of Hydrogen Chloride Gas
I. Physical Properties
5. Give the physical properties of HCl gas.
Ans. a) Colour – colourless.
b) Odour – pungent odour.
c) Taste – slightly sour.
d) Solubility – highly soluble in water.
e ) Density – it is 1.28 times heavier than air.
f) Physiological action : It is corrosive & attacks the respiratory passage causing burning sensation, it is non – poisonous.
6. How will you demonstrate the high solubility of hydrogen chloride by means of an experiment?
Ans. This can be shown by the FOUNTAIN EXPERIMENT. A round bottomed flask is filled with HCl gas. Take a double holed rubber cork and through one hole pass a dropper containing water & through the other a jet tube. On squeezing the dropper, water comes out and dissolves the HCl gas. This creates a partial vacuum and a low pressure inside the flask. The out side pressure is higher and pushes the blue litmus solution through jet tube into the round bottom flask. Here it combines with HCl gas and turns blue litmus red.

7. Answer the following questions with respect to the experiment shown in the figure .
(a) Name the experiment.
Ans. Fountain experiment.
(b) What does the experiment demonstrate with reference to HCl gas.
Ans. HCl gas is highly soluble in water and is acidic in nature.
(c) State the colour of water that has entered the round bottom flask.
Ans. Red.

8. How would you experimentally show that hydrogen chloride gas is heavier than air?
Ans.

Procedure : Take a dry gas jar filled with dry HCl gas and an empty gas jar. Place a burning candle in the empty gas jar and pour the HCl gas from the dry gas jar into the lower jar containing the burning candle.
Observation : The burning candle extinguishes.
Conclusion : HCl gas displaces the air from the lower jar and hence the candle is extinguished. Hence, HCl is heavier than air.
II Chemical Properties
9. Comment about the combustibility of HCl gas.
Ans. HCl gas is non – combustible and a non-supporter of combustion.
10. What would happen on heating HCl gas?
Ans. HCl gas dissociates reversibly into hydrogen and chlorine on heating above 500ºC.

11. Give two tests for HCl gas. Give balanced chemical equations for the reaction.
Ans. (a) When a glass rod dipped in ammonia is brought near HCl gas, it forms dense white fumes of ammonium chloride.
NH3 (g) + HCl (g) → NH4Cl (s)
(b) On heating active metals with hydrogen chloride gas, a gas is liberated which burns with a pale blue flame and a pop sound.

12. What is the colour change observed when HCl gas is introduced to the following indicators.
(a) Moist blue litmus paper.
(b) Methyl orange solution.
(c) Phenolphthalein solution.
(d) Alkaline phenolphthalein solution.
Ans. (a) Moist blue litmus turns red.
(b) Methyl orange solution turns from orange to pink.
(c) Phenolphthalein solution remains colourless.
(d) Alkaline phenolphthalein solution turn from pink to colourless.
13. It is necessary to use a moist litmus paper to test for acidity of HCl gas.
OR
Dry HCl gas has no effect on dry litmus paper but moist litmus paper turns red. Explain.
Ans. Dry HCl gas is covalent and does not have the presence of H+ ion. It therefore has no effect on dry red litmus. However HCl dissolves in polar solvents like water it ionises into H+ ion and Cl– ion.

It is the presence of H+ ions, which are responsible for the acidic properties, and turns blue litmus red.
14. (a) HCl is a monobasic acid. Explain.
Ans. One molecule of HCl produces only one H+ ion on dissociation, therefore it is monobasic.
HCl→ H+ + Cl– .
D. Hydrochloric acid and its preparation
Dissolution of HCl in water or Preparation of HCl acid
- 15. Answer the following questions with respect to preparation of hydrochloric acid fron hydrogen chloride gas.

(a) Name of the apparatus used for preparation of hydrochloric acid from hydrogen chloride gas. Or How will you dissolve HCl in water?
Ans. Special funnel arrangement.
(b) State the advantages of the above apparatus.
Ans. The advantages of the arrangement are :
a. It prevents the back suction of water into the apparatus.
b. It provides a larger surface area for dissolution of hydrogen chloride gas.
(c) In dissolving HCl in water why is a delivery tube not used ?
Ans. HCl is highly soluble in water, it dissolves at a faster rate than it is produced, thereby creating partial vacuum. The pressure outside the tube is higher and pushes the water up the tube. This is called BACK SUCTION and it prevents HCl from further dissolving in water and may damage the apparatus.
16. State the difference between hydrogen chloride gas and Hydrochloric acid.
Ans.

17 (a) How would you further concentrate the acid obtained?
Ans. The acid can be concentrated by boiling of the water.
(b) State why dilute HCl cannot be concentrated beyond a certain concentration by Boiling.
Ans. A solution of dilute HCl forms a constant boiling mixture, which on further boiling, boils without change in its composition evolving same proportion as its mixture. Hence dilute HCl cannot be concentrated by distilling or boiling the dilute acid.
18. What is constant boiling mixture?
Ans. When dil. HCl is heated and concentrated at 109.8ºC, it acquires a concentration of 22.2 % by weight on further heating both acid and water boil off and there is no further change in concentration. This is called as a constant boiling mixture.
19. Give the balanced chemical equations for the following reactions.
(a) Action of HCl on sodium carbonate.
Ans. Na2CO3 + 2HCl → 2NaCl + H2O + CO2 ↑
dil.
(b) HCl gas is passed through potassium carbonate solution.
Ans. K2CO3 + 2HCl → 2KCl + H2O + CO2 ↑
(c) Convert copper carbonate to copper chloride.
Ans. CuCO3 + 2HCl → CuCl2 + H2O + CO2 ↑
dil.
(d) Action of hydrochloric acid on calcium bicarbonate.
Ans. Ca(HCO3)2 +2HCl → CaCl2 + 2H2O + 2CO2 ↑
dil.
(e) Reaction taking place when HCl gas is passed through sodium hydroxide solution.
Ans. NaOH + HCl → NaCl + H2O
(f) Convert calcium oxide to calcium chloride.
Ans. CaO + 2HCl → CaCl2 + H2O
(g) Preparation of Magnesium chloride using hydrochloric acid.
Ans. Mg (OH)2 + 2HCl → MgCl2 + 2H2O OR
MgO + 2HCl → MgCl2 + H2O
(h) Action of dilute HCl on sodium sulphite.
Ans. Na2SO3 + 2HCl → 2NaCl + H2O + SO2 ↑
dil.
(i) Convert magnesium sulphite to magnesium chloride.
Ans. MgSO3 + 2HCl → MgCl2 + H2O + SO2 ↑
dil.
(j) Action of dil. HCl on potassium bisulphite.
Ans. KHSO3 + HCl → KCl + H2O + SO2 ↑
dil.
(k) Action of dilute HCl on lead sulphide.
Ans. PbS + 2HCl → PbCl2 + H2S ↑
dil.
(l) Convert zinc sulphide to zinc chloride.
Ans. ZnS + 2HCl → ZnCl2 + H2S ↑
dil.
(m) Oxidation of HCl using MnO2 as oxidising agent.

(r) Action of HCl on lead nitrate
Ans. Pb (NO3)2 + 2HCl → PbCl2 ↓ (White precipitate) + 2HNO3 dil.
(s) Action of HCl on silver nitrate.
Ans. AgNO3 + HCl → AgCl ↓ (White precipitate) + HNO3 dil.
(t) Action of dil HCl on Sodium thiosulphate
Ans. Na2S2O3 + 2HCl → 2NaCl + H2O + SO2 + S↓
dil.
20 (a) What is Aqua Regia? [Royal water]
Ans. Aqua Regia is made up of 3 parts of conc. HCl and 1 part of conc. HNO3.
Nascent chlorine is given out as follows:

Noble metals like gold and platinum do not dissolve in acids but they dissolve in Aqua Regia.
Aqua Regia reacts with gold and platinum to form soluble chlorides.
Pt + 4[Cl] → PtCl4
Au + 3[Cl] → AuCl3
(b) State why aquaregia dissolves gold, which is insoluble in all other acids.
Ans. Aquaregia, gives out nascent chlorine which dissolves gold. Hence, aquaregia dissolves gold which is insoluble in all other acids.
21. Give 3 tests for hydrochloric acid.
Ans. (i) A glass rod dipped in ammonia solution when brought near the mouth of a bottle containing conc. HCl gives out dense while fumes of NH4Cl.

When conc. HCl is added to oxidizing agents like black MnO2, lead dioxide, red lead, potassium permanganate or potassium dichromate, a greenish yellow, pungent smelling gas is evolved which turns moist blue litmus red & then bleaches it. It is chlorine. (Oxidising agents oxidize conc. HCl and liberate nascent chlorine.)
E. Uses of Hydrochloric acid
a. To extract glue from bones.
b. HCl is used in the production of dyes, drugs, paints, photographic chemicals etc.
c. To prepare aqua regia (a mixture of conc. Nitric acid and conc. Hydrochloric acid in the ratio 1 : 3 by volume) to dissolve noble metals like gold and platinum.
d. HCl acid is used to clear the metal surfaces, i.e. to remove the metallic oxide coating (Pickling), before coating the metal with zinc (Galvanizing).
e . It is used for the production of glucose from starch. (C6H10O5)n
f. HCl acid is used in the preparation of chlorides.
g. It is used as a laboratory reagent.
h. It is produced in the digestive tract of man and animals and helps in the digestion of food.
ADDITIONAL QUESTIONS
I. Name the following :
1. Common name for Hydrochloric acid – Muriatic acid.
2. Common name for hydrogen chloride – Spirit of salt.
3. Acidic constituents of gastric juices – Hydrochloric acid.
4. Two volatile acids – Hydrochloric acid, Nitric acid.
5. A non – volatile acid – Sulphuric acid.
6. An undesirable compound formed during the preparation of HCl above 200ºC. –Sodium sulphate.
7. An insoluble chloride – Silver chloride.
8. Two colourless gases which combine to form a solid – Ammonia and hydrogen chloride.
9. A solution used to test HCl acid – Silver nitrate.
10. A chloride soluble in hot water & insoluble in cold water – Lead chloride.
11. The solvent for silver chloride – Ammonium hydroxide.
12. The solvent for gold & platinum – Aqua Regia.
13. A gas which gives dense white fumes with hydrochloric acid – Ammonia.
14. A metal insoluble in mineral acids but soluble in aqua-regia – Gold and platinum.
15. A gas, which is neutral to dry litmus but turns moist blue litmus red – Hydrogen chloride.
16. Salt of a weak acid – Sodium carbonate.
17. A weak acid – Carbonic acid.
18. A trivalent metal chloride – Aluminium chloride / Ferric chloride.
19. A metal which liberates H2 explosively – Sodium and Potassium.
20. A basic gas, NH3 , reacts with an acidic gas, HCl, to form a white solid – Ammonium chloride
21. A gaseous covalent compound which dissolves in water & conducts electricity – Hydrogen chloride or Ammonia.
22. Two metals which do not displace H2 from acid – Cu, Hg, Ag, Au, Pt.
23. Two metals which displace H2 from acid – Zn, Mg, Fe.
24. A gas which is non – combustible & non a supporter of combustion – Hydrogen chloride.
25. A blue green chloride – Copper (II) Chloride.
26. An element which reacts with hydrogen to form a compound which is strongly acidic in water – Chlorine.
27. A black metallic oxide which reacts with hydrochloric acid to give a coloured solution and gas – Manganese dioxide
28. The experiment which demonstrates that hydrogen chloride is soluble in water– Fountain experiment.
29. The salt which gives a white ppt. an reacting with dil HCl, which is soluble in hot water – Lead nitrate Pb(NO3)2
30. The component responsible for the high dissolving power in aqua regia – Nascent Chlorine.
II. Complete the statements given below pertaining to hydrogen chloride gas or hydrochloric acid.
1. Hydrogen chloride gas is not dried using…………………………… [conc. H2SO4, CaO].
Ans. 1. CaO
2. Hydrogen chloride gas on heating above 500oC gives hydrogen and chlorine. The reaction is an example of ……………………………[thermal decomposition, thermal dissociation]
Ans. Thermal dissociation
3. Iron reacts with hydrogen chloride gas forming ……………………………[iron [II] chloride, iron [III] chloride] and hydrogen. The reaction is an example of [double decomposition, synthesis, simple displacement].
Ans. Iron (II) chloride; Simple displacement
4. Hydrogen chloride and water are examples of…………………………… [polar covalent compounds, non-polar covalent compounds]
Ans. Polar covalent compounds
5. Addition of _ [sodium nitrate, zinc nitrate, silver nitrate] to hydrochloric acid, gives an insoluble precipitate of the respective chloride. This precipitate is …………………………… [soluble, insoluble] in ammonium hydroxide & insoluble in dilute nitric acid.
Ans. Silver nitrate; soluble
6. Addition of ……………………………[iron [II] sulphide, iron [III] sulphide, iron pyrites] to dilute hydrochloric acid results in liberation of hydrogen sulphide gas.
Ans. Iron (II) sulphide
7. Hydrochloric acid can be converted into chlorine by heating with _ [calcium oxide, lead [III] oxide, lead [IV] oxide] which acts as a/an …………………………… [oxidising, reducing] agent.
Ans. Lead (IV) oxide, oxidising
III. Reactions:-
1. Basic gas to a white soluble solid
Ans. NH3 + HCl → NH4Cl
(Basic gas) (White soluble solid)
2. Hydrochloric acid → nascent chloride
Ans. HNO3 + 3HCl → 2H2O + NOCl + 2[Cl]
3. Soluble metallic nitrate → insoluble metallic chloride
Ans. Pb(NO3)2 + HCl → PbCl2 ↓(White precipitate) + 2HNO3
dil.
4. Iron → Iron (II) chloride
Ans. Fe + HCl → FeCl2 + H2
dil.
5. Soluble metal nitrate → insoluble metal chloride → Soluble salt
Ans. AgNO3 + HCl → AgCl ↓ + HNO3
dil.
AgCl+ 2NH4OH → Ag(NH3)2Cl + 2H2O
6. Sodium thiosulphate → Sodium chloride
Ans. Na2S2O3+ 2HCl → 2NaCl + H2O + SO2+ S
dil.
7. Black metal oxide → greenish yellow gas
Ans. MnO2 + 4HCl → MnCl2 + 2H2O + Cl2↓
conc.
(b) Study the flow chart and give balanced equations with conditions for the conversions A , B, and D.

Ans.

IV) Answer the following.
1. Solution A reacts with an acid B to given an acidic gas which gives greenish yellow gas on reacting with oxidising agents like Pb3O4 . The gas on passing through AgNO3 solution gives white precipitate C insoluble in nitric acid but soluble in ammonium hydroxide. Name A, B and C.
Ans. A :- Sodium chloride (NaCl)
B :- Conc. Sulphuric acid.
C :- Silver chloride (AgCl)
2. An acidic gas X (litmus Blue – Red) combines with an alkaline gas Y (litmus Red – Blue) to form a white solid Z. Identify X, Y, Z and give the balanced chemical equation.
Ans. For the reaction,
X = HCl, Y = NH3 , Z = NH4Cl
Reaction is as follows

3. MNO2 , PbO2 and red lead react with conc. HCl and liberates Cl2. What is the common property being shown by these metal oxides.
Ans. They are oxidising agents.
4. Giving reason state which of the two a solution of HCl in water or in toluene is an electrolyte.
Ans. A solution of HCl in water is an electrolyte, water is a polar covalent solvent on dissolving HCl in it, HCl ionises to give H+and Cl- ions which conduct electricity. Whereas, a solution of HCl in toluene, a non-polar solvent does not ionise HCl and hence does not conduct electricity.
5. Explain :-
(a) We do not prepare silver nitrate solution from tap water.
Ans. Tap water contains chlorine. This forms dil. hydrochloric acid, which will form a white ppt. with AgNO3 soln. Hence tap water is not used.
(b) AgNO3 is used to test for chloride, and not zinc nitrate.
Ans. HCl does not normally react with nitrates and thus will show no visible reaction with Zn(NO3)2
HCl, however reacts with AgNO3
AgNO3 + HCl → AgCl ↓ + HNO3: This gives a white ppt. which is visible and thus helps in identification.
(c) HCl acid is used to clean metal surfaces (pickling) before galvanizing.
Ans. HCl acid is used to clean metal surfaces (pickling) before galvanizing as it removes oxide layer formed over the metal.
(d) Dry hydrogen chloride gas does not affect a dry strip of blue litmus paper but it turns red in the presence of water.
Ans. H+ ions formed on ionisation of hydrogen chloride gas results in its acidic property. The ionisation occurs in the presence of a polar covalent solvent such as water. Hence, dry HCl gas does not affect a dry strip of blue litmus
paper but it turns red in the presence of water
(e) When the stopper of a bottle full of hydrogen chloride gas is opened there are fumes in the air.
Ans. Due to the high solubility of HCl gas in water, it fumes in moist air forming tiny droplets of hydrochloric acid.
6. What will you observe when.
(a) HCl gas is passed through silver nitrate solution?
Ans. A white precipitate is formed.
(b) HCl gas comes in contact with ammonia solution?
Ans. Dense white fumes are seen.
(c) HCl gas is passed through lead nitrate solution and the product is heated?
Ans. A white ppt. is formed which gets dissolved on heating.
(d) Platinum is added to Aqua Regia?
Ans. It dissolves.
(e) CuO is treated with dil. HCl acid?
Ans. Black colour CuO dissolves in it to give a blue colour solution.
(f) Manganese dioxide is added to Conc. HCl.
Ans. Gives a greenish yellow gas.
(g) Dil HCl is added to a carbonate salt (Na2CO3).
Ans. Effervescence is seen liberating a gas which turns lime water milky.
(h) Dil HCl is added to a sulphite salt (Na2SO3).
Ans. A colourless gas is evolved which turns acidified KMnO4 solution pink to clear colourless.
(i) Dil HCl is added to sulphide salt (Na2S) .
Ans. A colourless gas with a rotten egg smell is evolved.
(j) Dil HCl is added to Sodium thiosulphate .
Ans. A yellow precipitate of sulphur is observed and a gas is evolved which turns lime water milky & acidified KMnO4 pink to clear colourless
7. Using HCl how will you distinguish between the following?
(a) PbO2 and PbO
Ans. PbO2 + 4HCl → PbCl2 + 2H2O + Cl2↓
conc.
PbO2 liberates Cl2, a greenish yellow gas, turns litmus – blue to red and then bleaches it.
PbO + 2HCl → PbCl2 + H2O; Chlorine gas is not evolved.
conc.
(b) AgNO3 and Pb(NO3)2 Ans. AgNO3 + HCl → white ppt of AgCl, insoluble in acid soluble in NH4OH.
dil.
Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3 :
dil.
These give white crystals which are soluble in hot water.
(c) Na2S (sulphide) & Na2SO3 (sulphite)
Ans. Na2S + 2HCl → 2NaCl + H2S ↓ gives out a gas with the smell of rotten
dil.
egg and turns lead acetate paper silvery black due to the formation of lead sulphide.
Na2SO3 + 2HCl → 2NaCl + H2O + SO2 ↓
dil.
Gives out a gas with a pungent suffocating odour. This turns potassium permanganate solution from pink to colourless and acidified potassium dichromate paper turns from orange to green showing that the gas evolved is SO2.
(d) Fe and copper :
Ans. On addition of dil. HCl, Fe will give out a gas which burns with a pop sound.
Fe + 2HCl → FeCl2 + H2 ↓
dil.
Cu there will be no reaction.
(e) CuO and ZnO.
Ans. On addition of dil. HCl
CuO + 2HCl → CuCl2 + H2O
CuO forms a blue coloured solution, which on addition of NaOH forms a pale blue ppt.
ZnO + 2HCl → ZnCl2 + H2O
ZnO – forms a colourless soln. When NaOH is added, it forms a gelatinous white ppt. soluble in excess.
SUMMARY OF REACTIONS
a. Preparation of Hydrogen chloride

b. Properties of Hydrogen chloride

Na2CO3 + 2HCl → 2NaCl + H2O + CO2 ↓
K2CO3 + 2HCl → 2KCl + H2O + CO2 ↓
CuCO3 + 2HCl → CuCl2 + H2O + CO2 ↓
Ca(HCO3)2 + 2HCl → CaCl2 + 2H2O + 2CO2 ↓
NaOH + HCl → NaCl + H2O
CaO + 2HCl → CaCl2 + H2O
Mg (OH)2 + 2HCl → MgCl2 + 2H2O
MgO + 2HCl → MgCl2 + H2O
Na2SO3 + 2HCl → 2NaCl + H2O + SO2 ↓
MgSO3 + 2HCl → MgCl2 + H2O + SO2 ↓
KHSO3 + HCl → KCl + H2O + SO2 ↓
PbS + 2HCl → PbCl2 + H2S ↓
ZnS + 2HCl → ZnCl2 + H2S ↓
c. Reaction with oxidising agents
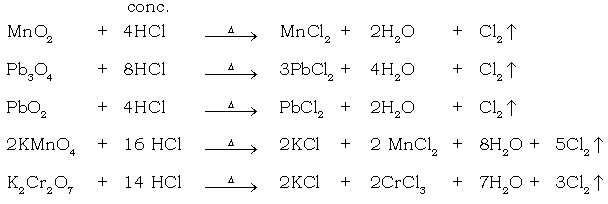
d. Test for hydrochloric acid
AgNO3 + HCl → HNO3 + AgCl ↓
dil.



