Students of ICSE Class 10 should refer to Ammonia ICSE Class 10 Chemistry Questions below which have come in past board exams. You should always go through questions that have come in previous years. This will help you to understand the pattern of questions in ICSE Class 10 Chemistry and prepare accordingly. This will help you to get better marks in ICSE Class 10 Board Exams
ICSE Class 10 Chemistry Important Questions Ammonia
Ammonia is an important chapter in ICSE Class 10 Chemistry. Our faculty has prepared the following ICSE Class 10 Chemistry Questions and answers based on the latest syllabus and books issued for the current academic year. Please refer to the solved questions below and also see links provided for other chapters.
Ammonia ICSE Class 10 Chemistry Questions
Ammonia ICSE Class 10 Chemistry Questions
Introduction
• Ammonia was initially called ‘alkaline air’. It occurs in both free and in the chemically combined state. Small quantities of ammonia occurs in atmospheric air and natural water in free state.
• In the combined state ammonia occurs in the form of ammonium salts and ammonium liquor.
• Molecular formula of ammonia is NH3.
• Relative molecular mass of NH3 is 17.
A. Preparation of Ammonia
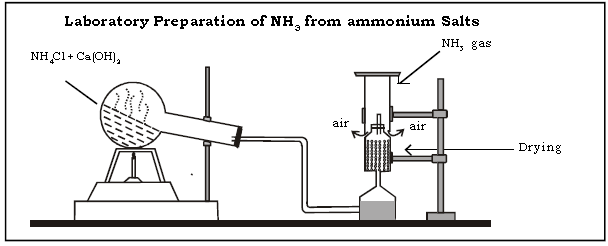
(i) Laboratory preparation
1. Answer the following questions pertaining to preparation of ammonia in the laboratory using ammonium salts.
a. Name the reactants used in the above preparation.
Ans. The reactants are sal ammoniac or ammonium chloride (NH4Cl) and slaked lime or calcium hydroxide [Ca(OH)2]
b. Give the balanced chemical equation for the reaction.
Ans.

c. In the preparation of Ammonia gas why solid Ammonium chloride and slaked lime are taken in the ratio 2 : 3 by weight.
Ans. Solid ammonium chloride is a sublimable solid which could be lost during direct heating. The higher ratio of slaked lime is used to counteract the loss of Ammonium chloride by sublimation.
d. Why Ammonium nitrate is not used in the preparation of Ammonia?
Ans. Ammonium nitrate is not used since it is explosive in nature and may itself decompose forming nitrous oxide and water vapour.
e. Why is calcium hydroxide preferred over other caustic alkalis. (NaOH & KOH)
Ans. Calcium hydroxide is preferred over other caustic alkalis, because it is cheap and not deliquescent.
f. How is ammonia gas dried?
Ans. It is dried using the drying agent, quick lime (CaO), which is basic and does not react with NH3 which is also basic.
g. Why ammonia gas is not dried by using drying agents like conc. sulphuric acid calcium chloride and phosphorous pentoxide?
Ans. Drying agents like conc. sulphuric acid, calcium chloride and phosphorous pentoxide are not used for drying because all these drying agents react with ammonia.
2NH3 + H2SO4 → (NH4)2SO4
(conc.) ammonium sulphate
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
ammonium phosphate
8NH3 + CaCl2 → CaCl2
.8NH3
(fused) addition product
h. How is ammonia gas collected?
Ans. Ammonia gas is lighter than air and hence collected by the downward displacement of air.
i. Why ammonia gas cannot be collected over water?
Ans. It is not collected over water since it is highly soluble in water (1 vol. of water dissolves about 702 vols. at 20ºC and 1 atmospheric pressure.)
j. Why is the apparatus kept in a tilted/inclined position?
Ans. The apparatus is kept in a tilted or inclined position to provide maximum surface area for the reaction and so that the water vapour formed may not trickle back and crack the hot flask.
h. How would you determine whether the gas jar is filled completely with ammonia?
Ans. A glass rod dipped in HCl is brought near the mouth of the gas jar, if dense white fumes are seen, we can conclude that the gas jar is filled with ammonia.
2. Give the balanced chemical equation for the following reaction.
(a) Conversion of ammonium chloride to ammonia.
Ans.

(b) Preparation of ammonia using ammonium sulphate and sodium hydroxide.
Ans.

(c) Reaction of ammonium chloride and sodium hydroxide.
Ans.

3. Answer the following with respect to preparation of ammonia from metal nitrides.

(a) Give the equation for preparation of following metal nitrides.
(i) Mg3N2 (ii) Ca3N2 & (iii) AlN
Ans. (i) 3Mg + N2 → Mg3 N2
(ii) 3Ca + N2 → Ca3N2
(iii) 2Al + N2 → 2AlN
(b) Give the balanced chemical equation for the preparation of ammonia using the following metal nitrides.
(i) Mg3N2 (ii) Ca3N2 (iii) AlN
Ans. (i) Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3(g) ↓
warm.
(ii) Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3 ↓
warm.
(iii) AlN + 3H2O → Al(OH)3 + NH3↓
warm.
4. Answer the following questions with respect to manufacture of ammonia.

(a) Name the process used for manufacture of ammonia.
Ans. Haber’s process.
(b) (i) Name the reactants used.
(ii) State the ratio in which the reactants are used.
(iii) How are the above reactants obtained in pure state.
Ans. (i) The reactants used are nitrogen & hydrogen.
(ii) They are added in a ratio of 1 : 3 i.e 1 volume of nitrogen & 3 volumes of hydrogen.
(iii) Pure nitrogen is obtained by fractional distillation of liquid air. Pure hydrogen is obtained from water gas by Bosch process.
(c) Give the balanced chemical equation for the reaction.
Ans.
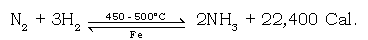
(d) State the conditions required to carry out the above reaction.
Ans. i. Temperature – 450 – 500ºC (optimum temp.)
ii. Pressure – 200 – 900 atm.
iii. Catalyst – Finely divided iron
iv. Promoter – Molybdenum (Mo) / Al2O3
The above reaction is reversible, exothermic and proceeds with a decrease in volume. Low temperature and high pressure favours the forward reaction.
(e) Why is the reaction temperature in the above process around 450ºC to 500ºC?
Ans. According to the Chatelier’s principle, if a reaction is exothermic the forward reaction is favoured by lowering the temperature therefore an optimum temperature of 450ºC – 500ºC is kept.
(f) Why should the catalyst be free from impurities?
Ans. Impurities like CO, CO2 and H2S poison the catalyst and hence the nitrogen hydrogen reactant mixture must be free from them.
(g) What is the function of catalyst and promoter?
Ans. The catalyst speeds up the reaction but does not increase the yield of ammonia whereas promoter enhances the efficiency of the catalyst.
(h) State the two physical properties of ammonia which can be used to separate ammonia from unreacted nitrogen and hydrogen?
Ans. The two properties of ammonia are :-
(i) Ammonia is easily liquefiable where as N2 and H2 are difficult to liquefy.
(ii) Ammonia is highly soluble in water, whereas N2 and H2 are almost insoluble in water.
(i) Give the two techniques used for separating ammonia from uncombined nitrogen and hydrogen.
Ans. Liquefaction and dissolution in water.
(j) How is ammonia seperated from nitrogen and hydrogen?
Ans. Mixture of NH3 along with residual nitrogen and hydrogen under pressure is allowed to expand suddenly through a small nozzle into a region of low pressure. This results in the fall in temperature thereby liquifying easily liquefiable NH3 gas while nitrogen and Hydrogen are difficult to liquify.
B. Properties of Ammonia
5. Give an experiment to show the high solubility of ammonia gas in water.
Ans. The high solubility of NH3 gas is demonstrated by the fountain experiment.
(i) An inverted flask containing dry NH3 gas is arranged as shown in the diagram.
(ii) When the dropper containing water is squeezed, the water enters the flask and since NH3 gas is highly soluble in water, it dissolves creating a partial vaccum in the flask.

(iii) The outside pressure being higher than the inside pressure it forces the red litmus solution to move upwards through the jet tube emerging out as a blue fountain.
6. Why an aqueous solution of ammonia is basic in nature?
Ans. The aqueous solution of ammonia (NH4OH) acts as a weak base, since it undergoes partial dissociation in aqueous solution to give hydroxyl ions (OH–) in low concentration.
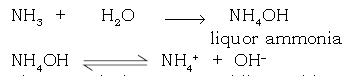
The aq. solution turns red litmus blue and phenolphthalein solution pink.
7. What is liquor ammonia?
Ans. An aqueous solution of ammonia in water is called liquor ammonia or liquor ammonia fortis (saturated solution specific gravity 0.88).
NH3 + H2O →NH4OH
(liquor ammonia)
8. Give the balanced chemical equation of the following reaction.
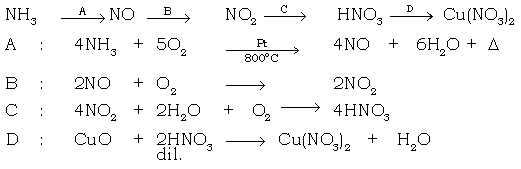
(a) Burning of ammonia in air
Ans. 4NH3 + 3O2 → 2N2 + 6H2O
(b) Catalytic oxidation of ammonia.
Ans.

(c) Two ways of preparing ammonium chloride using HCl.
Ans. NH3 + HCl → NH4Cl
NH4OH + HCl → NH4Cl + H2O
(d) Preparation of ammonium sulphate from ammonia.
Ans. 2NH3 + H2SO4 → (NH4)2SO4
(e) Conversion of ammonium hydroxide to ammonium sulphate.
Ans. 2NH4OH + H2SO4 → (NH4)2SO4 + 2H2O
(f) Convert nitric acid to ammonium nitrate.
Ans. NH4OH + HNO3 → NH4NO3 + H2O
(g) Reduction of copper oxide using ammonia.
Ans. 3CuO + 2NH3 → 3 Cu + 3H2O + N2↓
(Basic oxide)
(h) Convert yellow coloured oxide to its corresponding metal.
Ans. 3PbO + 2NH3 → 3Pb + 3H2O + N2↓
(Amphoteric oxide)
(i) Reduction of chlorine using ammonia.
Ans. 2NH3 + 3Cl2 → N2 + 6HCl
(j) Reduction of chloride using excess of ammonia.
Ans. 8NH3 + 3Cl2 → N2 + 6NH4Cl
excess
(k) Action of excess of chlorine on ammonia.
Ans. NH3 + 3Cl2 → 3HCl + NCl3 [Nitrogen tri chloride]
excess
9. Why is an aqueous solution of ammonia used for identifying cations?
Ans. On dissolving ammonia in water, it forms ammonium hydroxide. Ammonium hydroxide reacts with metallic salt solutions to give insoluble precipitates of the respective metallic hydroxides which vary in colour & solubility in
excess NH4OH. Hence, ammonium hydroxide is used for identifying cations.
10. State how NH4OH is used for identifying.
(i) Fe2+, (ii) Fe3+, (iii) Pb2+, (iv) Zn2+, (v) Cu2+ cations. Also give balanced equation in each case.
Ans. (i) On adding ammonium hydroxide to iron (II) sulphate, a dirty green ppt. of ferrous hydroxide is obtained which is insoluble in excess ammonium hydroxide.
FeSO4 + 2NH4OH → (NH4)2 SO4 + Fe(OH)2 ↓
(dirty green precipitate)
ferrous hydroxide
(ii) On adding ammonium hydroxide to ferric chloride, a reddish brown Ppt of ferric hydroxide is obtained which is insoluble in excess ammonium hydroxide.
FeCl3 + 3NH4OH → 3NH4Cl+ Fe(OH)3 ↓ [Reddish brown precipitate]
Ferric hydroxide
(iii) On adding ammonium hydroxide to lead nitrate solution, a chalky white precipitate of lead hydroxide is obtained which is insoluble in excess ammonium hydroxide.
Pb(NO3)2 + 2NH4OH → 2NH4NO3 + Pb(OH)2 ↓ [chalky white precipitate]
Lead hydroxide
(iv) On adding ammonium hydroxide to zinc nitrate solution, a gelatinous white precipitate of zinc hydroxide is obtained which is soluble in excess ammonium hydroxide.
Zn(NO3)2 + 2NH4OH → 2NH4NO3 + Zn(OH)2 ↓ [White gelatinous precipitate]
Zinc hydroxide
(v) On adding ammonium hydroxide to copper sulphate solution a pale blue precipitate of copper is obtained which gets soluble in excess of ammonium hydroxide forming a deep blue or inky blue solution.
CuSO4 + 2NH4OH →(NH4)2SO4 + Cu(OH)2 ↓ [Pale blue precipitate]
Copper hydroxide
Cu(OH)2 + (NH4)2SO4 + 2NH4OH → [Cu(NH3)4]SO4 + 4H2O
Tetrammine copper (II) sulphate
(Deep blue or inky blue solution)
C. Tests and Uses of Ammonia
11. Give some important tests for ammonia gas.
Ans. (i) It turns moist red litmus blue.
(ii) A glass rod dipped in conc. HCl acid brought near ammonia gas, dense white fumes of ammonium chloride are formed.
(iii) Ammonia gas passed through Nesseler’s reagent gives brown coloured precipitate.
(iv) Ammonia gas passed through copper sulphate solution gives a pale blue precipitate of copper hydroxide, which turns into a deep blue coloured solution due to the formation of soluble complex tetramine copper (II) sulphate.
12. Give few large scale or industrial uses of ammonia.
Ans. (i) Manufacture of fertilizers e.g. urea, ammonium sulphate, ammonium nitrate, ammonium phosphate etc.
(ii) In the manufacture of important ammonium compounds such as :
a) Ammonium chloride used in dry cells, medicine and textile industry and cleaning metal surfaces.
b) Ammonium carbonate (constituent of smelling salt) helps in reviving
a fainted person and is also used in baking and dyeing industry.
c) Ammonium sulphate is used in the manufacture of alum.
13. Give two uses of Liquor ammonia.
Ans. 1. It is used as a cleansing agent. It emulsifies or dissolves fats, grease stains from clothes.
2. It is also used for cleaning window panes, porcelain articles etc.
14. What would you observe when ammonia gas is passed through following indicators.
(a) Moist red litmus paper.
(b) Methyl orange solution
(c) Phenolphthalein solution.
Ans. (a) Moist red litmus paper turns blue.
(b) Methyl orange turns from orange to yellow.
(c) Phenolphthalein solution turns from colourless to pink.
ADDITIONAL QUESTIONS
I. Name the following.
1. The solution of ammonia in water.
Ans. Liquor ammonia.
2. The ion other than ammonium ion present in aqueous solution of ammonia.
Ans. Hydroxyl (OH–).
3. A black coloured compound which turns reddish brown on passing ammonia gas.
Ans. Copper oxide.
4. A yellow coloured liquid formed when excess of chloride is passed through ammonia.
Ans. Nitrogen trichloride.
5. A gas which is prepared by Haber’s process.
Ans. Ammonia.
6. A salt of ammonia used in dry cells.
Ans. Ammonium chloride.
7. A metallic chloride soluble in ammonium hydroxide.
Ans. Potassium chloride or sodium chloride.
8. The gas obtained when ammonia burns in an atmosphere of oxygen without any catalyst .
Ans. Nitrogen.
9. A nitride of a divalent metal which reacts with warm water liberating ammonia.
Ans. Magnesium nitride or calcium nitride.
10. An acid obtained from ammonia.
Ans. Nitric acid
11. Drying agent for ammonia gas.
Ans. Quicklime.
12. Catalyst used in Haber’s process.
Ans. Finely divided iron.
13. Two solutions which react together to produce nitrogen.
Ans. Ammonium chloride (NH4Cl) and sodium nitrite NaNO2.
14. Metal which directly combines with nitrogen on heating.
Ans. Magnesium, calcium, aluminium.
15. An amphoteric oxide reduced by NH3 (g).
Ans. Lead (II) oxide (PbO).
16. Two gases which gives dense white fumes with ammonia.
Ans. Chlorine (Cl2) and Hydrogen chloride (HCl) (g).
17. The solution which turns brown when it comes in contact with ammonia.
Ans. Nessler’s reagent.
18. Experiment which demonstrate the extreme solubility of ammonia.
Ans. Fountain experiment.
19. A colourless gas which becomes reddish brown when it comes in contact with atmosphere (oxygen).
Ans. Nitric oxide NO.
20. An amphoteric oxide reduced by NH3 (g).
Ans. Lead (II) oxide (PbO).
II. Complete the statements by selecting the correct word from the words in brackets.
1. The alkaline behaviour of liquor ammonia is due to the presence of ____________ions. [ammonium/ hydronium / hydroxyl]
Ans. hydroxyl.
2. Ammonia reduces chlorine to __________[nitrogen / hydrogen chloride/ ammonium chloride]
Ans. Hydrogen chloride.
3. On heating ammonia and oxygen in the presence of platinum _________ (nitrogen/nitrogen monoxide) gas is produced.
Ans. Nitrogen monoxide.
4. Ammonia burns in air with a ______(greenish yellow / reddish brown) coloured flame.
Ans. Greenish yellow.
5. Ammonium chloride is a soluble salt prepared by _________[precipitation, neutralisation].
Ans. Neutralisation.
6. When ammonium chloride is heated, it undergoes thermal ___________. (decomposition / dissociation).
Ans. Dissociation.
7. Heating ammonium chloride with sodium hydroxide produces ___________ [ammonia, nitrogen].
Ans. ammonia.
III. Correct the following statements.
1. A reddish brown precipitate is obtained when ammonium hydroxide is added to ferrous sulphate.
Ans. A reddish brown precipitate is obtained when ammonium hydroxide is added to ferric sulphate.
Or
A dirty green precipitate is obtained when ammonium hydroxide is added to ferrous sulphate.
2. Liquid ammonia is an aqueous solution of NH3.
Ans. Liquor ammonia is an aqueous solution of NH3.
3. Finely divided platinum is used in Haber’s Process.
Ans. Finely divided iron is used in Haber’s process.
4. Conc. H2SO4 is a drying agent for NH3.
Ans. CaO is a drying agent for NH3
5. Ammonium chloride on decomposition gives nitrous oxide.
Ans. Ammonium nitrate on decomposition gives nitrous oxide or Ammonium chloride on decomposition gives ammonia.
IV. Select the most probable substance from A,B,C,D & E which need to be added to distinguish:
[a -Conc. hydrochloric acid, b-Ammonia gas, c – Barium chloride, d – Phenolphthalein, e- Sodium hydroxide]
1. Ammonium sulphate and ammonium chloride.
Ans. c- Barium chloride.
2. Potassium sulphate and ammonium sulphate.
Ans. e- Sodium hydroxide
3. Liquor ammonia and liquid ammonia
Ans. d- Phenolphthalein
4. Ammonia and sulphur dioxide gas
Ans. a- Conc. hydrochloric acid
5. Copper (II) oxide and copper (II) chloride
Ans. b- Ammonia gas
V.1. Give reasons for the following:
1. Liquid ammonia is used as a refrigerant in ice plants.
Ans. Ammonia is environmentally compatible. It does not deplete ozone layer and does not contribute to global warming. It has superior thermodynamic qualities as a result ammonia refrigeration systems use less electricity.
Ammonia has a recognizable odour and so leaks are not likely to escape.
2. Aqueous solution of ammonia is used for removing grease stains from woollen clothes.
Ans. Liquor ammonia emulsifies or dissolves fats, grease etc. Hence it is used for removing grease stains from woollen clothes.
3. Aqueous solution of ammonia conducts electricity.
Ans. In its aqueous solution ammonia is present in the form of NH4OH, which dissociate partially to give NH4
+ & OH– ions which are responsible for conduction of electricity.
4. Ammonium compounds do not occur as minerals.
Ans. Ammonia and ammonium compounds are highly soluble in water and hence they do not occur as minerals.
5. Why an optimum pressure of 200 – 900 atm. is used in the Haber’s process?
Ans. According to Le Chatelier’s principle if a reaction proceeds with decrease in volume then the forward reaction is favoured (i.e. higher percentage yield of NH3) by increasing the pressure.
VI. Chemical equations.
1. How will you convert i) Mg ii) Ca iii) Al to ammonia? Give equation only.
Ans. (i) 3Mg + N2 → Mg3N2
Magnesium nitrogen magnesium nitride
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3(g)
Magnesium water magnesium Ammonia
nitride (warm) hydroxide
(ii) 3Ca + N2 → Ca3N2
Calcium Nitrogen Calcium nitride
Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3(g)
Calcium warm Calcium Ammonia
nitride water hydroxide
(iii) 2Al + N2 → 2AlN
Aluminium Nitrogen Aluminium nitride
AlN + 3H2O → Al(OH)3 + NH3(g)
Aluminium warm Aluminium Ammonia
nitride water hydroxide
2. Give balanced equations for the following conversions:
(a) Ammonia to brown gas.
Ans.

(b) Ammonia to nitrogen trichloride,
Ans. NH3 + 3Cl2 (excess) → 3HCl + NCl3
(c) Ammonia solution to an amphoteric hydroxide
Ans. Pb(NO3)2 + 2NH4OH → Pb(OH)2 ↓ + 2NH4NO3
OR
Zn(SO4) + 2NH4OH → Zn(OH)2 ↓ + (NH4)2SO4
(d) A nitride of trivalent metal to ammonia.
Ans. AlN + 3H2O → Al(OH)3 + NH3
warm
(e) Lead oxide to lead.
Ans. 3PbO + 2NH3 → 3Pb + N2 + 3H2O
3. Write the equation for the action of heat on:
(a) Ammonium chloride, (b) Ammonium nitrate.
state whether each reaction is an example of thermal decomposition or thermal dissociation.
Ans. (a) Ammonium chloride:
NH4Cl → NH3 + HCl → Thermal dissociation
Ans. b) Ammonium nitrate:
NH4NO3 → Δ N2O(g) + 2H2O → Thermal decomposition
OR
NH3HCl → NH4Cl.
4. Give balanced equations for the following conversions – A,B, C, D & E.

Ans. (i) A: 2NH4OH + H2SO4 → (NH4)2SO4 + H2O
B: (NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3 ↓
(ii) C: NH3 + HCl → NH4Cl
D: 8NH3 + 3Cl2 → 6NH4Cl + N2
E: 4NH3 + 3O2 → 2N2 + 6H2Ollowing conversions – A,B, C, D & E.
5. Give equation for the following conversions.
Ans.
6. Give equation for the following conversions.
Ans.
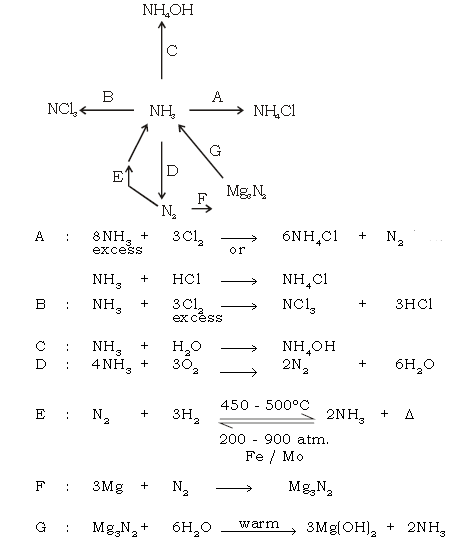
7. Give equation for the following conversions.

Ans.
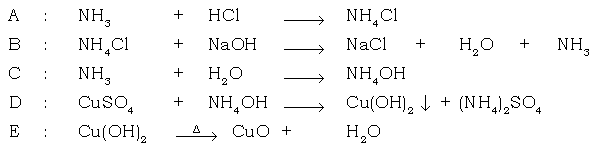
VIII. Answer the following :
1. Name an ammonium salt which is a constituent of smelling salt and give reason for its use.
Ans. Ammonium carbonate is a constituent of smelling salt.
It dissociates evolving pungent ammonia gas and revives a fainted person.
2. A white crystalline compound N when warmed with concentrated sulphuric acid gives a gas which fumes in moist air and forms dense white fumes with ammonia. When N is warmed with sodium hydroxide solution, a gas is evolved which turns moist red litmus blue.
a) Name each of the gases evolved.
b) What is N?
Ans: a) Hydrogen chloride gas is evolved which gives dense white fumes of ammonium chloride with ammonia.
When N is warmed with sodium hydroxide solution, ammonia gas is evolved.
b) N is ammomium chloride (NH4Cl).
3. Answer the following questions with respect to the diagram given below.
(a) Name the gases passed through A & B
Ans. A :- Ammonia gas B :- Oxygen gas.
(b) What is observed when the dry gas A is burnt alone.
Ans. The gas does not burn.
(c) What is the inference of the above observation .
Ans. Ammonia gas is neither combustible nor a supporter of combustion.
(d) What is observed when gas A is burnt in the presence of gas B.
Ans. Ammonia burns in the presence of oxygen (gas B) with a greenish yellow flame.
(e) Give the balanced chemical equation of the above reaction.
Ans. 4NH3 + 3O2 → 2N2 + 6H2O
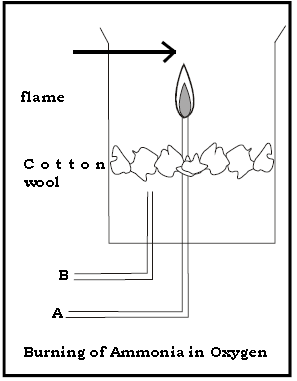
4. Answer the following questions with respect to the diagram given below.

(a) In what ratio is dry ammonia & dry oxygen gas added.
Ans. 1: 2
(b) Give the equation for the reaction taking place.
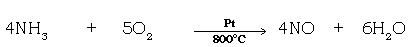
(c) Name the gas collected in flask C.
Ans. Nitrogen monoxide.
(d) What is the colour change observed in flask C ? why ?
Ans. The colourless gas collected turns reddish brown.
Because nitrogen monoxide gets oxidised to give reddish brown coloured nitrogen dioxide.
2NO + O2 → 2NO2
(e) The platinum catalyst continues to glow even after heating is discontinued.
Ans. The platinum catalyst continues to glow, since the catalytic oxidation of ammonia is an exothermic process.
5. Give an equation of reaction which proves that ammonia contains nitrogen and hydrogen.
Ans. 3CuO + 2NH3 → 3 Cu + 3H2O + N2 ↓
VIII. Observation based questions:-
(a) Ammonia gas bubbled through red litmus solution.
Ans. Red litmus soln turns blue.
(b) Ammonia burns in oxygen.
Ans. It burns with greenish yellow flame.
(c) Ammonia is passed over heated copper oxide.
Ans. Black coloured copper oxide changes to pinkish red metal.
(d) Ammonia is passed over heated Lead (II) oxide
Ans. Buff yellow lead (II) oxide is reduced to greyish metallic lead.
(e) When NH3 gas in excess is mixed with chlorine.
Ans. Dense white fumes are seen.
(f) When NH3 gas is passed through neutral litmus solution.
Ans. Neutral litmus (purple) turns blue in alkaline solution.
(g) Ammonia is passed through Nessler’s reagent first little then in excess.
Ans. Colourless Nesseler’s reagent turns brown, on passing excess gas brown precipitate is obtained.
(h) Ammonia is passed through copper sulphate first little, then in excess.
Ans. Pale blue precipitate is formed which solubilizes in excess of ammonia to give a deep blue coloured solution.
(i) Ammonia reacts with excess of chlorine.
Ans. A yellow coloured explosive liquid is obtained.
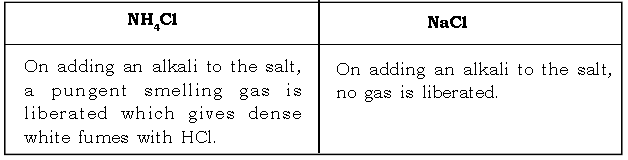
2. Give a chemical test to distinguish between ammonium chloride and sodium chloride.
Ans.
SUMMARY OF EQUATIONS
a. Preparation of Ammonia :
Laboratory preparation

b. Reactions of Ammonia with drying agents :
2NH3 + H2SO4 → (NH4)2 SO4
(conc)
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
8NH3 + CaCl2 → CaCl2. 8NH3
fused
c. Laboratory preparation :
From metal nitrides
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
warm
Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3
warm
AlN + 3H2O → Al(OH)3 + NH3
d. Manufacture of Ammonia :
Haber’s process

e. Chemical properties of ammonia :
(i) Combustibility
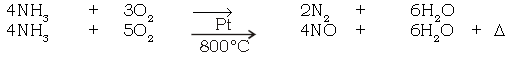
(ii) Basic nature of ammonia :
NH3 + HCl → NH4Cl
NH3 + HNO3 → NH4NO3
2NH3 + H2SO4 → (NH4)2SO4
NH4OH + HCl → NH4Cl + H2O
NH4OH + HNO3 → NH4NO3 + H2O
2NH4OH + H2SO4 → (NH4)2SO4 + 2H2O
(iii) Ammonia with metallic salt solution :
FeSO4 + 2NH4OH → (NH4)2SO4 + Fe(OH)2 ↓
FeCl3 + 3NH4OH → 3NH4Cl + Fe(OH)3 ↓
Pb(NO3)2 + 2NH4OH → 2NH4NO3 + Pb(OH)2 ↓
Zn(NO3)2 + 2NH4OH → 2NH4NO3 + Zn(OH)2 ↓
Zn(OH)2 + (NH4)2SO4 + 2NH4OH → [Zn(NH3)4]SO4 + 4H2O
CuSO4 + 2NH4OH → (NH4)2SO4 + Cu(OH)2 ↓
Cu(OH)2 + (NH4)2SO4 + 2NH4OH → [Cu(NH3)4]SO4 + 4H2O
f. Ammonia gas as a reducing agent
2NH3 + 3CuO → 3Cu + 3H2O + N2 ↑
2NH3 + 3PbO → 3Pb + 3H2O + N2 ↑
2NH3 + 3Cl2 → 6HCl + N2 ↑
NH3 + HCl → NH4Cl
8NH3 (excess) + 3Cl2 → 6NH4Cl + N2 ↑
NH3 + 3Cl2 → 3HCl + NCl3



